Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells
- PMID: 30733805
- PMCID: PMC6348814
- DOI: 10.1155/2019/3904645
Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells
Abstract
Aldehyde dehydrogenase (ALDH) is a superfamily of enzymes that detoxify a variety of endogenous and exogenous aldehydes and are required for the biosynthesis of retinoic acid (RA) and other molecular regulators of cellular function. Over the past decade, high ALDH activity has been increasingly used as a selectable marker for normal cell populations enriched in stem and progenitor cells, as well as for cell populations from cancer tissues enriched in tumor-initiating stem-like cells. Mounting evidence suggests that ALDH not only may be used as a marker for stem cells but also may well regulate cellular functions related to self-renewal, expansion, differentiation, and resistance to drugs and radiation. ALDH exerts its functional actions partly through RA biosynthesis, as all-trans RA reverses the functional effects of pharmacological inhibition or genetic suppression of ALDH activity in many cell types in vitro. There is substantial evidence to suggest that the role of ALDH as a stem cell marker comes down to the specific isoform(s) expressed in a particular tissue. Much emphasis has been placed on the ALDH1A1 and ALDH1A3 members of the ALDH1 family of cytosolic enzymes required for RA biosynthesis. ALDH1A1 and ALDH1A3 regulate cellular function in both normal stem cells and tumor-initiating stem-like cells, promoting tumor growth and resistance to drugs and radiation. An improved understanding of the molecular mechanisms by which ALDH regulates cellular function will likely open new avenues in many fields, especially in tissue regeneration and oncology.
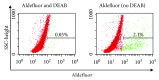
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

