The feasibility of fingerstick blood collection for point-of-care HIV-1 viral load monitoring in rural Zambia
- PMID: 30734030
- PMCID: PMC6363362
- DOI: 10.15641/ghi.v1i2.585
The feasibility of fingerstick blood collection for point-of-care HIV-1 viral load monitoring in rural Zambia
Abstract
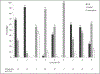
Viral load monitoring for HIV treatment is recommended but not feasible in many settings. A point-of-care test using capillary blood would increase access but may require up to 200 μL of blood to achieve a lower limit of detection of 1000 copies/mL. This cross-sectional study evaluated the feasibility of collecting 200 μL of capillary blood and blood collection preferences among adults in rural Zambia. Adults seeking HIV counseling and testing at Macha Hospital were recruited in 2015. Capillary blood was collected in four 50 μL tubes. Blood collection was categorized as complete (200 μL collected), partial (all tubes filled but <200 μL obtained due to collection techniques), or incomplete (1-4 tubes attempted; <200 μL obtained due to insufficient blood flow). One fingerstick was required for 90% of the 201 participants. A median blood volume of 196 μL was collected. Complete, partial and incomplete collection was achieved in 34%, 59% and 6% of participants. The majority of participants (95%) preferred fingerstick over venous blood collection. A point-of-care viral load test requiring up to 200 μL of blood is feasible in a rural setting but would require training and supervision to ensure that sufficient blood was collected.
Keywords: HIV; capillary blood; point-of-care test; sub-Saharan Africa; viral load testing.
Figures

References
-
- BOILLOT F, SERRANO L, MUWONGA J, KABUAYI JP, KAMBALE A, MUTAKA F, FUJIWARA PI, DECOSAS J, PEETERS M & DELAPORTE E 2016. Implementation and operational research: Programmatic feasibility of dried blood spots for the virological follow-up of patients on antiretroviral treatment in Nord Kivu, Democratic Republic of the Congo. J Acquir Immune Defic Syndr, 71, e9–15. - PMC - PubMed
-
- LOFGREN SM, MORRISSEY AB, CHEVALLIER CC, MALABEJA AI, EDMONDS S, AMOS B, SIFUNA DJ, VON SEIDLEIN L, SCHIMANA W, STEVENS WS, BARTLETT JA & CRUMP JA 2009. Evaluation of a dried blood spot HIV-1 RNA program for early infant diagnosis and viral load monitoring at rural and remote healthcare facilities. AIDS, 23, 2459–66. - PMC - PubMed
-
- MAIERS TJ, GOUS N, NDUNA M, MCFALL SM, KELSO DM, FISHER MJ, PALAMOUNTAIN KM, SCOTT LE & STEVENS WS 2015. An investigation of fingerstick blood collection for point-of-care HIV-1 viral load monitoring in South Africa. S Afr Med J, 105, 228–31. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
