Real-World Experience with Tofacitinib in IBD at a Tertiary Center
- PMID: 30734234
- PMCID: PMC6935176
- DOI: 10.1007/s10620-019-05492-y
Real-World Experience with Tofacitinib in IBD at a Tertiary Center
Abstract
Background and aims: Many inflammatory bowel disease (IBD) patients do not respond to medical therapy. Tofacitinib is a first-in-class, partially selective inhibitor of Janus kinase, recently approved for treating patients with ulcerative colitis (UC). We describe our experience with the use of tofacitinib for treatment of patients with moderate-to-severe IBD.
Methods: This is a retrospective, observational study of the use of tofacitinib in IBD. Patients with medically resistant IBD were treated orally with 5 mg or 10 mg twice daily. Clinical response and adverse events were assessed at 8, 26, and 52 weeks. Objective response was assessed endoscopically, radiologically, and biochemically.
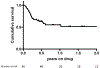
Results: 58 patients (53 UC, 4 Crohn's, 1 pouchitis) completed at least 8 weeks of treatment with tofacitinib. 93% of the patients previously failed treatment with anti-TNF. At 8 weeks of treatment, 21 patients (36%) achieved a clinical response, and 19 (33%) achieved clinical remission. Steroid-free remission at 8 weeks was achieved in 15 patients (26%). Of the 48 patients followed for 26 weeks, 21% had clinical, steroid-free remission. Of the 26 patients followed for 12 months, 27% were in clinical, steroid-free remission. Twelve episodes of systemic infections were noted, mostly while on concomitant steroids. One episode of herpes zoster infection was noted during follow-up.
Conclusions: In this cohort of patients with moderate-to-severe, anti-TNF resistant IBD, tofacitinib induced clinical response in 69% of the patients. 27% were in clinical, steroid-free remission by 1 year of treatment. Tofacitinib is an effective therapeutic option for this challenging patient population.
Keywords: Inflammatory bowel disease; Jak inhibitors; Tofacitinib; Ulcerative colitis.
Figures
References
-
- Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of Inflammatory Bowel Disease Among Adults Aged >/=18 Years - United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65(42):1166–1169. - PubMed
-
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46–54 e42; quiz e30. - PubMed
-
- Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ 2017;357:j2505. - PubMed
-
- Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011;106(4):644–659, quiz 660. - PubMed
-
- Amiot A, Serrero M, Peyrin-Biroulet L, et al. One-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther 2017;46(3):310–321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical