Assessment of neuropeptide binding sites and the impact of biostable kinin and CAP2b analogue treatment on aphid (Myzus persicae and Macrosiphum rosae) stress tolerance
- PMID: 30734498
- PMCID: PMC6593983
- DOI: 10.1002/ps.5372
Assessment of neuropeptide binding sites and the impact of biostable kinin and CAP2b analogue treatment on aphid (Myzus persicae and Macrosiphum rosae) stress tolerance
Abstract
Background: Neuropeptides are regulators of critical life processes in insects and, due to their high specificity, represent potential targets in the development of greener insecticidal agents. Fundamental to this drive is understanding neuroendocrine pathways that control key physiological processes in pest insects and the screening of potential analogues. The current study investigated neuropeptide binding sites of kinin and CAPA (CAPA-1) in the aphids Myzus persicae and Macrosiphum rosae and the effect of biostable analogues on aphid fitness under conditions of desiccation, starvation and thermal (cold) stress.
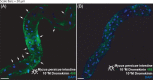
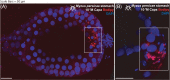
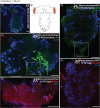
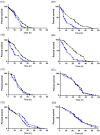
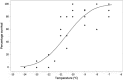
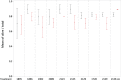
Results: M. persicae and M. rosae displayed identical patterns of neuropeptide receptor mapping along the gut, with the gut musculature representing the main target for kinin and CAPA-1 action. While kinin receptor binding was observed in the brain and VNC of M. persicae, this was not observed in M. rosae. Furthermore, no CAPA-1 receptor binding was observed in the brain and VNC of either species. CAP2b/PK analogues (with CAPA receptor cross-activity) were most effective in reducing aphid fitness under conditions of desiccation and starvation stress, particularly analogues 1895 (2Abf-Suc-FGPRLa) and 2129 (2Abf-Suc-ATPRIa), which expedited aphid mortality. All analogues, with the exception of 2139-Ac, were efficient at reducing aphid survival under cold stress, although were equivalent in the strength of their effect.
Conclusion: In demonstrating the effects of analogues belonging to the CAP2b neuropeptide family and key analogue structures that reduce aphid fitness under stress conditions, this research will feed into the development of second generation analogues and ultimately the development of neuropeptidomimetic-based insecticidal agents. © 2019 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: G-protein coupled receptors receptor-mapping; aphicide; cold tolerance; desiccation; ligand-binding; pest control.
© 2019 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Figures

References
-
- Pimentel D, Acquay H, Biltonen M, Rice P, Silva M, Nelson J et al., Environmental and economic costs of pesticide use. Bioscience 42:750–760 (1992).
-
- Wilson C and Tisdell C, Why farmers continue to use pesticides despite environmental, health and sustainability costs. Ecol Econ 39:449–462 (2001).
-
- Altstein M and Nässel DR, Neuropeptide signaling in insects. Adv Exp Med Biol 692:155–165 (2010). - PubMed
-
- Nachman RJ and Pietrantonio PV, Interaction of mimetic analogs of insect kinin neuropeptides with arthropod receptors. Adv Exp Med Biol 692:27–48 (2010). - PubMed
-
- Audsley N and Down RE, G protein coupled receptors as targets for next generation pesticides. Insect Biochem Molec 67:27–37 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

