Effect Modification by Baseline Mortality in the MORDOR Azithromycin Trial
- PMID: 30734696
- PMCID: PMC7470539
- DOI: 10.4269/ajtmh.18-1004
Effect Modification by Baseline Mortality in the MORDOR Azithromycin Trial
Abstract
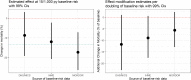
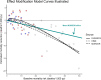
We examined whether baseline mortality risk, as a function of child age and site, modified the azithromycin mortality-reduction effect in the Macrolide Oraux pour Réduire les Décès avec un Oeil sur la Résistance (MORDOR) clinical trial. We used the Cox proportional hazards model with an interaction term. Three models were examined representing three sources for the baseline-risk covariate: two using sources external to MORDOR and the third leveraging data within MORDOR. All three models provided moderate evidence for the effect becoming stronger with increasing baseline mortality (P = 0.02, 0.02, and 0.07, respectively) at the rate of approximately 6-12% additional mortality reduction per doubling of baseline mortality. Etiological and programmatic implications of these findings are discussed.
Figures


References
-
- DHS , 2017. Malawi Demographic and Health Survey 2015–16, Final Report. Available at: https://dhsprogram.com/publications/publication-fr319-dhs-final-reports.cfm. Accessed December 11, 2018.
-
- DHS , 2016. Tanzania Demographic and Health Survey and Malaria Indicator Survey, 2015–16, Final Report. Available at: https://dhsprogram.com/publications/publication-FR321-DHS-Final-Reports.cfm. Accessed December 11, 2018.
-
- MICS , 2013. Enquête Démographique et de Santé et à Indicateurs Multiples (EDSN-MICS IV) 2012 (French). Available at: http://mics.unicef.org/surveys. Accessed December 11, 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

