Prostate brachytherapy with iodine-125 seeds: analysis of a single institutional cohort
- PMID: 30735336
- PMCID: PMC6541135
- DOI: 10.1590/S1677-5538.IBJU.2018.0142
Prostate brachytherapy with iodine-125 seeds: analysis of a single institutional cohort
Abstract
Objectives: Brachytherapy (BT) with iodine-125 seeds placement is a consolidated treatment for prostate cancer. The objective of this study was to assess the clinical outcomes in patients with prostate cancer who underwent low-dose-rate (LDR) -BT alone in a single Brazilian institution.
Materials and methods: Patients treated with iodine-125 BT were retrospectively assessed after at least 5 years of follow-up. Patients who received combination therapy (External beam radiation therapy-EBRT and BT) and salvage BT were not included.
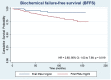
Results: 406 men were included in the study (65.5% low-risk, 30% intermediate-risk, and 4.5% high-risk patients). After a median follow-up of 87.5 months, 61 (15.0%) patients developed biochemical recurrence. The actuarial biochemical failure-free survival (BFFS) at 5 and 10 years were 90.6% and 82.2%, respectively. A PSA nadir ≥ 1 ng / mL was associated with a higher risk of biochemical failure (HR = 5.81; 95% CI: 3.39 to 9.94; p ≤ 0.001). The actuarial metastasis-free survival (MFS) at 5 and 10 years were 98.3% and 94%, respectively. The actuarial overall survival (OS) at 5 and 10 years were 96.2% and 85.1%, respectively. Acute and late grade 2 and 3 gastrointestinal toxicities were observed in 5.6%, 0.5%, 4.6% and 0.5% of cases, respectively. For genitourinary the observed acute and late grade 2 and 3 toxicities rates were 57.3%, 3.6%, 28% and 3.1%, respectively. No grade 4 and 5 were observed.
Conclusions: BT was effective as a definitive treatment modality for prostate cancer, and its endpoints and toxicities were comparable to those of the main series in the literature.
Keywords: Brachytherapy; Iodine-125 [Supplementary Concept]; Prostate.
Copyright® by the International Brazilian Journal of Urology.
Conflict of interest statement
None declared.
Figures




References
-
- Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. - PMC - PubMed
-
- Kupelian PA, Potters L, Khuntia D, Ciezki JP, Reddy CA, Reuther AM, et al. Radical prostatectomy, external beam radiotherapy < 72Gy, external beam radiotherapy > or =72Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:25–33. - PubMed
-
- Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375:1415–1424. - PubMed
-
- Critz FA, Benton JB, Shrake P, Merlin ML. 25-Year disease-free survival rate after irradiation for prostate cancer calculated with the prostate specific antigen definition of recurrence used for radical prostatectomy. J Urol. 2013;189:878–883. - PubMed
-
- Grimm P, Billiet I, Bostwick D, Dicker AP, Frank S, Immerzeel J, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109(Suppl 1):22–29. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
