TRPA1 channel contributes to myocardial ischemia-reperfusion injury
- PMID: 30735434
- PMCID: PMC6483018
- DOI: 10.1152/ajpheart.00106.2018
TRPA1 channel contributes to myocardial ischemia-reperfusion injury
Abstract
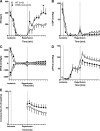
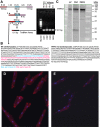
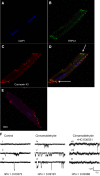
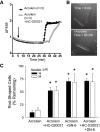
Myocardial ischemia-reperfusion (I/R) results in the generation of free radicals, accumulation of lipid peroxidation-derived unsaturated aldehydes, variable angina (pain), and infarction. The transient receptor potential ankyrin 1 (TRPA1) mediates pain signaling and is activated by unsaturated aldehydes, including acrolein and 4-hydroxynonenal. The contribution of TRPA1 (a Ca2+-permeable channel) to I/R-induced myocardial injury is unknown. We tested the hypothesis that cardiac TRPA1 confers myocyte sensitivity to aldehyde accumulation and promotes I/R injury. Although basal cardiovascular function in TRPA1-null mice was similar to that in wild-type (WT) mice, infarct size was significantly smaller in TRPA1-null mice than in WT mice (34.1 ± 9.3 vs. 14.3 ± 9.9% of the risk region, n = 8 and 7, respectively, P < 0.05), despite a similar I/R-induced area at risk (40.3 ±8.4% and 42.2 ± 11.3% for WT and TRPA1-null mice, respectively) after myocardial I/R (30 min of ischemia followed by 24 h of reperfusion) in situ. Positive TRPA1 immunofluorescence was present in murine and human hearts and was colocalized with connexin43 at intercalated disks in isolated murine cardiomyocytes. Cardiomyocyte TRPA1 was confirmed by quantitative RT-PCR, DNA sequencing, Western blot analysis, and electrophysiology. A role of TRPA1 in cardiomyocyte toxicity was demonstrated in isolated cardiomyocytes exposed to acrolein, an I/R-associated toxin that induces Ca2+ accumulation and hypercontraction, effects significantly blunted by HC-030031, a TRPA1 antagonist. Protection induced by HC-030031 was quantitatively equivalent to that induced by SN-6, a Na+/Ca2+ exchange inhibitor, further supporting a role of Ca2+ overload in acrolein-induced cardiomyocyte toxicity. These data indicate that cardiac TRPA1 activation likely contributes to I/R injury and, thus, that TRPA1 may be a novel therapeutic target for decreasing myocardial I/R injury. NEW & NOTEWORTHY Transient receptor potential ankyrin 1 (TRPA1) activation mediates increased blood flow, edema, and pain reception, yet its role in myocardial ischemia-reperfusion (I/R) injury is unknown. Genetic ablation of TRPA1 significantly decreased myocardial infarction after I/R in mice. Functional TRPA1 in cardiomyocytes was enriched in intercalated disks and contributed to acrolein-induced Ca2+ overload and hypercontraction. These data indicate that I/R activation of TRPA1 worsens myocardial infarction; TRPA1 may be a potential target to mitigate I/R injury.
Keywords: acrolein; cardiomyocytes; lipid peroxidation; myocardial infarction; transient receptor potential ankyrin 1 channel; unsaturated aldehydes.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by α,β-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118: 2574–2582, 2008. doi:10.1172/JCI34886. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

