Control of basal autophagy rate by vacuolar peduncle
- PMID: 30735514
- PMCID: PMC6368412
- DOI: 10.1371/journal.pone.0209759
Control of basal autophagy rate by vacuolar peduncle
Abstract
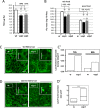
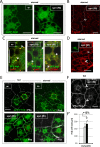
Basal autophagy is as a compressive catabolic mechanism engaged in the breakdown of damaged macromolecules and organelles leading to the recycling of elementary nutrients. Thought essential to cellular refreshing, little is known about the origin of a constitutional rate of basal autophagy. Here, we found that loss of Drosophila vacuolar peduncle (vap), a presumed GAP enzyme, is associated with enhanced basal autophagy rate and physiological alterations resulting in a wasteful cell energy balance, a hallmark of overactive autophagy. By contrast, starvation-induced autophagy was disrupted in vap mutant conditions, leading to a block of maturation into autolysosomes. This phenotype stem for exacerbated biogenesis of PI(3)P-dependent endomembranes, including autophagosome membranes and ectopic fusions of vesicles. These findings shed new light on the neurodegenerative phenotype found associated to mutant vap adult brains in a former study. A partner of Vap, Sprint (Spri), acting as an endocytic GEF for Rab5, had the converse effect of leading to a reduction in PI(3)P-dependent endomembrane formation in mutants. Spri was conditional to normal basal autophagy and instrumental to the starvation-sensitivity phenotype specific of vap. Rab5 activity itself was essential for PI(3)P and for pre-autophagosome structures formation. We propose that Vap/Spri complexes promote a cell surface-derived flow of endocytic Rab5-containing vesicles, the traffic of which is crucial for the implementation of a basal autophagy rate.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








References
-
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell [Internet]. 2008. January 11;132(1):27–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18191218 - PMC - PubMed
-
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev [Internet]. 2010;90(4):1383–435. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20959619 - PubMed
-
- Knævelsrud H, Simonsen A. Fighting disease by selective autophagy of aggregate-prone proteins. FEBS Lett [Internet]. 2010. June 18;584(12):2635–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20412801 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

