Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution
- PMID: 30735633
- PMCID: PMC6544371
- DOI: 10.1016/j.cell.2019.01.017
Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution
Abstract
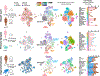
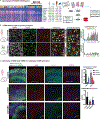
Direct comparisons of human and non-human primate brains can reveal molecular pathways underlying remarkable specializations of the human brain. However, chimpanzee tissue is inaccessible during neocortical neurogenesis when differences in brain size first appear. To identify human-specific features of cortical development, we leveraged recent innovations that permit generating pluripotent stem cell-derived cerebral organoids from chimpanzee. Despite metabolic differences, organoid models preserve gene regulatory networks related to primary cell types and developmental processes. We further identified 261 differentially expressed genes in human compared to both chimpanzee organoids and macaque cortex, enriched for recent gene duplications, and including multiple regulators of PI3K-AKT-mTOR signaling. We observed increased activation of this pathway in human radial glia, dependent on two receptors upregulated specifically in human: INSR and ITGB8. Our findings establish a platform for systematic analysis of molecular changes contributing to human brain development and evolution.
Keywords: cerebral organoids; chimpanzee; cortical development; human-specific evolution; mTOR; macaque; neural progenitor cells; radial glia; single-cell RNA sequencing.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

