Combining Slow Flow Techniques With Adaptive Demodulation for Improved Perfusion Ultrasound Imaging Without Contrast
- PMID: 30735994
- PMCID: PMC6528792
- DOI: 10.1109/TUFFC.2019.2898127
Combining Slow Flow Techniques With Adaptive Demodulation for Improved Perfusion Ultrasound Imaging Without Contrast
Abstract
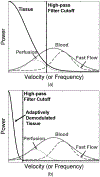
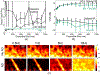
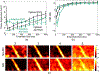
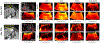
Noncontrast perfusion ultrasound imaging remains challenging due to spectral broadening of the tissue clutter signal caused by patient and sonographer hand motion. To address this problem, we previously introduced an adaptive demodulation scheme to suppress the bandwidth of tissue prior to high-pass filtering. Our initial implementation used single plane wave power Doppler imaging and a conventional tissue filter. Recent advancements in beamforming and tissue filtering have been proposed for improved slow flow imaging, including coherent flow power Doppler (CFPD) imaging and singular value decomposition (SVD) filtering. Here, we aim to evaluate adaptive demodulation in conjunction with improvements in beamforming and filtering using simulations, single-vessel phantoms, and an in vivo liver tumor embolization study. We show that simulated blood-to-background contrast-to-noise ratios are highest when using adaptive demodulation with CFPD and a 100-ms ensemble, which resulted in a 13.6-dB average increase in contrast-to-noise ratio compared to basic IIR filtering alone. We also show that combining adaptive demodulation with SVD and with CFPD + SVD results in 9.3- and 19-dB increases in contrast-to-noise ratios compared to IIR filtering alone at 700- and 500-ms ensembles for phantom data with 1- and 5-mm/s average flows, respectively. In general, combining techniques resulted in higher signal-to-noise, contrast-to-noise, and generalized contrast-to-noise ratios in both simulations and phantoms. Finally, adaptive demodulation with SVD resulted in the largest qualitative and quantitative changes in tumor-to-background contrast postembolization.
Figures

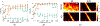



References
-
- Heimdal A and Torp H, “Ultrasound doppler measurements of low velocity blood flow: limitations due to clutter signals from vibrating muscles,” IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control, vol. 44, pp. 873–881, 1997.
-
- Tremblay-Darveau C, Williams R, Milot L, Bruce M, and Burns PN, “Combined perfusion and doppler imaging using plane-wave nonlinear detection and microbubble contrast agents,” IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control, vol. 61, pp. 1988–2000, 2014. - PubMed
-
- Jensen JA, Estimation of Blood Velocities Using Ultrasound: A Signal Processing Approach. Cambridge: Cambridge UP, 1996.
-
- Bjaerum S, Torp H, and Kristoffersen K, “Clutter filters adapted to tissue motion in ultrasound color flow imaging,” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, vol. 49, pp. 693–704, 2002. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

