Retro-1-Oligonucleotide Conjugates. Synthesis and Biological Evaluation
- PMID: 30736307
- PMCID: PMC6384773
- DOI: 10.3390/molecules24030579
Retro-1-Oligonucleotide Conjugates. Synthesis and Biological Evaluation
Abstract
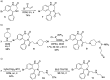
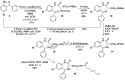
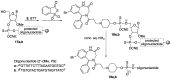
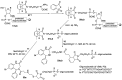
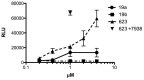
Addition of small molecule Retro-1 has been described to enhance antisense and splice switching oligonucleotides. With the aim of assessing the effect of covalently linking Retro-1 to the biologically active oligonucleotide, three different derivatives of Retro-1 were prepared that incorporated a phosphoramidite group, a thiol or a 1,3-diene, respectively. Retro-1⁻oligonucleotide conjugates were assembled both on-resin (coupling of the phosphoramidite) and from reactions in solution (Michael-type thiol-maleimide reaction and Diels-Alder cycloaddition). Splice switching assays with the resulting conjugates showed that they were active but that they provided little advantage over the unconjugated oligonucleotide in the well-known HeLa Luc705 reporter system.
Keywords: Retro-1; antisense; oligonucleotide conjugates; splice switching.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures










References
-
- Sharma V.K., Sharma R.K., Singh S.K. Antisense oligonucleotides: Modifications and clinical trials. Med. Chem. Commun. 2014;5:1454–1471. doi: 10.1039/C4MD00184B. - DOI

