The Role of Osteoprotegerin and Its Ligands in Vascular Function
- PMID: 30736365
- PMCID: PMC6387017
- DOI: 10.3390/ijms20030705
The Role of Osteoprotegerin and Its Ligands in Vascular Function
Abstract
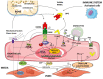
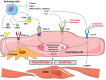
The superfamily of tumor necrosis factor (TNF) receptors includes osteoprotegerin (OPG) and its ligands, which are receptor activators of nuclear factor kappa-B ligand (RANKL) and TNF-related apoptosis-inducing ligand (TRAIL). The OPG/RANKL/RANK system plays an active role in pathological angiogenesis and inflammation as well as cell survival. It has been demonstrated that there is crosstalk between endothelial cells and osteoblasts during osteogenesis, thus establishing a connection between angiogenesis and osteogenesis. This OPG/RANKL/RANK/TRAIL system acts on specific cell surface receptors, which are then able to transmit their signals to other intracellular components and modify gene expression. Cytokine production and activation of their receptors induce mechanisms to recruit monocytes and neutrophils as well as endothelial cells. Data support the role of an increased OPG/RANKL ratio as a possible marker of progression of endothelial dysfunction in metabolic disorders in relationship with inflammatory marker levels. We review the role of the OPG/RANKL/RANK triad in vascular function as well as molecular mechanisms related to the etiology of vascular diseases. The potential therapeutic strategies may be very promising in the future.
Keywords: OPG/RANKL/RANK; endothelium; osteoprotegerin; vascular disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Barbu C.G., Arsene A.L., Florea S., Albu A., Sirbu A., Martin S., Nicolae A.C., Burcea-Dragomiroiu G.T.A., Popa D.E., Velescu B.S., et al. Cardiovascular risk assessment in osteoporotic patients using osteoprotegerin as a reliable predictive biochemical marker. Mol. Med. Rep. 2017;16:6059–6067. doi: 10.3892/mmr.2017.7376. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

