CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception
- PMID: 30736422
- PMCID: PMC6386987
- DOI: 10.3390/ijms20030711
CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception
Abstract
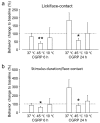
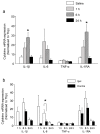
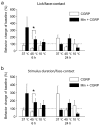
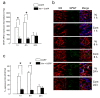
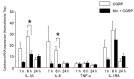
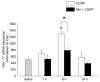
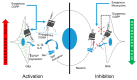
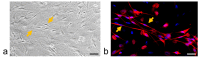
Neuron-glia interactions contribute to pain initiation and sustainment. Intra-ganglionic (IG) secretion of calcitonin gene-related peptide (CGRP) in the trigeminal ganglion (TG) modulates pain transmission through neuron-glia signaling, contributing to various orofacial pain conditions. The present study aimed to investigate the role of satellite glial cells (SGC) in TG in causing cytokine-related orofacial nociception in response to IG administration of CGRP. For that purpose, CGRP alone (10 μL of 10-5 M), Minocycline (5 μL containing 10 μg) followed by CGRP with one hour gap (Min + CGRP) were administered directly inside the TG in independent experiments. Rats were evaluated for thermal hyperalgesia at 6 and 24 h post-injection using an operant orofacial pain assessment device (OPAD) at three temperatures (37, 45 and 10 °C). Quantitative real-time PCR was performed to evaluate the mRNA expression of IL-1β, IL-6, TNF-α, IL-1 receptor antagonist (IL-1RA), sodium channel 1.7 (NaV 1.7, for assessment of neuronal activation) and glial fibrillary acidic protein (GFAP, a marker of glial activation). The cytokines released in culture media from purified glial cells were evaluated using antibody cytokine array. IG CGRP caused heat hyperalgesia between 6⁻24 h (paired-t test, p < 0.05). Between 1 to 6 h the mRNA and protein expressions of GFAP was increased in parallel with an increase in the mRNA expression of pro-inflammatory cytokines IL-1β and anti-inflammatory cytokine IL-1RA and NaV1.7 (one-way ANOVA followed by Dunnett's post hoc test, p < 0.05). To investigate whether glial inhibition is useful to prevent nociception symptoms, Minocycline (glial inhibitor) was administered IG 1 h before CGRP injection. Minocycline reversed CGRP-induced thermal nociception, glial activity, and down-regulated IL-1β and IL-6 cytokines significantly at 6 h (t-test, p < 0.05). Purified glial cells in culture showed an increase in release of 20 cytokines after stimulation with CGRP. Our findings demonstrate that SGCs in the sensory ganglia contribute to the occurrence of pain via cytokine expression and that glial inhibition can effectively control the development of nociception.
Keywords: calcitonin gene related peptide; cytokine; satellite glial cells; thermal hyperalgesia; trigeminal ganglion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

