Bats and Viruses: Emergence of Novel Lyssaviruses and Association of Bats with Viral Zoonoses in the EU
- PMID: 30736432
- PMCID: PMC6473451
- DOI: 10.3390/tropicalmed4010031
Bats and Viruses: Emergence of Novel Lyssaviruses and Association of Bats with Viral Zoonoses in the EU
Abstract
: Bats in the EU have been associated with several zoonotic viral pathogens of significance to both human and animal health. Virus discovery continues to expand the existing understating of virus classification, and the increased interest in bats globally as reservoirs or carriers of zoonotic agents has fuelled the continued detection and characterisation of new lyssaviruses and other viral zoonoses. Although the transmission of lyssaviruses from bat species to humans or terrestrial species appears rare, interest in these viruses remains, through their ability to cause the invariably fatal encephalitis-rabies. The association of bats with other viral zoonoses is also of great interest. Much of the EU is free of terrestrial rabies, but several bat species harbor lyssaviruses that remain a risk to human and animal health. Whilst the rabies virus is the main cause of rabies globally, novel related viruses continue to be discovered, predominantly in bat populations, that are of interest purely through their classification within the lyssavirus genus alongside the rabies virus. Although the rabies virus is principally transmitted from the bite of infected dogs, these related lyssaviruses are primarily transmitted to humans and terrestrial carnivores by bats. Even though reports of zoonotic viruses from bats within the EU are rare, to protect human and animal health, it is important characterise novel bat viruses for several reasons, namely: (i) to investigate the mechanisms for the maintenance, potential routes of transmission, and resulting clinical signs, if any, in their natural hosts; (ii) to investigate the ability of existing vaccines, where available, to protect against these viruses; (iii) to evaluate the potential for spill over and onward transmission of viral pathogens in novel terrestrial hosts. This review is an update on the current situation regarding zoonotic virus discovery within bats in the EU, and provides details of potential future mechanisms to control the threat from these deadly pathogens.
Keywords: bats; emerging; lyssavirus; novel; rabies; zoonoses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
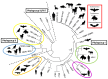
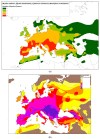
References
-
- Banyard A.C., Fooks A.R. The impact of novel lyssavirus discovery. Microbiol. Aust. 2017;38:18–21. doi: 10.1071/MA17006. - DOI
-
- Marston D.A., Horton D.L., Nunez J., Ellis R.J., Orton R.J., Johnson N., Banyard A.C., McElhinney L.M., Freuling C.M., Firat M., et al. Genetic analysis of a rabies virus host shift event reveals within-host viral dynamics in a new host. Virus Evol. 2017;3:vex038. doi: 10.1093/ve/vex038. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

