In Vitro Activity of the Bacteriophage Endolysin HY-133 against Staphylococcus aureus Small-Colony Variants and Their Corresponding Wild Types
- PMID: 30736446
- PMCID: PMC6387228
- DOI: 10.3390/ijms20030716
In Vitro Activity of the Bacteriophage Endolysin HY-133 against Staphylococcus aureus Small-Colony Variants and Their Corresponding Wild Types
Abstract
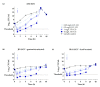
Nasal carriage of methicillin-susceptible (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) represents both a source and a risk factor for subsequent infections. However, existing MRSA decolonization strategies and antibiotic treatment options are hampered by the duration of administration and particularly by the emergence of resistance. Moreover, beyond classical resistance mechanisms, functional resistance as the formation of the small-colony variant (SCV) phenotype may also impair the course and treatment of S. aureus infections. For the recombinant bacteriophage endolysin HY-133, rapid bactericidal and highly selective in vitro activities against MSSA and MRSA has been shown. In order to assess the in vitro efficacy of HY-133 against the SCV phenotype, minimal inhibitory (MIC) and minimal bactericidal concentrations (MBC) were evaluated on clinical SCVs, their isogenic wild types, as well as on genetically derived and gentamicin-selected SCVs. For all strains and growth phases, HY-133 MIC and MBC ranged between 0.12 and 1 mg/L. Time-kill studies revealed a fast-acting bactericidal activity of HY-133 resulting in a ≥3 - log10 decrease in CFU/mL within 1 h compared to oxacillin, which required 4⁻24 h. Since the mode of action of HY-133 was independent of growth phase, resistance pattern, and phenotype, it is a promising candidate for future S. aureus decolonization strategies comprising rapid activity against phenotypic variants exhibiting functional resistance.
Keywords: HY-133; Staphylococcus aureus; endolysin; functional resistance; nasal decolonization; oxacillin; small-colony variants; susceptibility.
Conflict of interest statement
S.M. and H.G. are employees of HYpharm GmbH. The other authors declare no conflict of interest.
Figures


References
-
- Köck R., Becker K., Cookson B., van Gemert-Pijnen J.E., Harbarth S., Kluytmans J., Mielke M., Peters G., Skov R.L., Struelens M.J., et al. Methicillin-resistant Staphylococcus aureus (MRSA): Burden of disease and control challenges in Europe. Euro Surveill. 2010;15:19688. doi: 10.2807/ese.15.41.19688-en. - DOI - PubMed
-
- Wertheim H.F., Vos M.C., Ott A., van Belkum A., Voss A., Kluytmans J.A., van Keulen P.H., Vandenbroucke-Grauls C.M., Meester M.H., Verbrugh H.A. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364:703–705. doi: 10.1016/S0140-6736(04)16897-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

