Collagen Fiber Array of Peritumoral Stroma Influences Epithelial-to-Mesenchymal Transition and Invasive Potential of Mammary Cancer Cells
- PMID: 30736469
- PMCID: PMC6406296
- DOI: 10.3390/jcm8020213
Collagen Fiber Array of Peritumoral Stroma Influences Epithelial-to-Mesenchymal Transition and Invasive Potential of Mammary Cancer Cells
Abstract
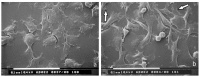
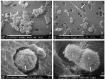
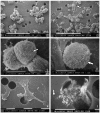
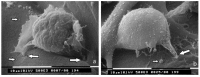
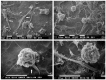
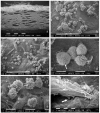
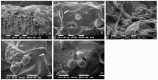
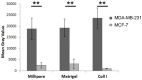
: Interactions of cancer cells with matrix macromolecules of the surrounding tumor stroma are critical to mediate invasion and metastasis. In this study, we reproduced the collagen mechanical barriers in vitro (i.e., basement membrane, lamina propria under basement membrane, and deeper bundled collagen fibers with different array). These were used in 3D cell cultures to define their effects on morphology and behavior of breast cancer cells with different metastatic potential (MCF-7 and MDA-MB-231) using scanning electron microscope (SEM). We demonstrated that breast cancer cells cultured in 2D and 3D cultures on different collagen substrates show different morphologies: i) a globular/spherical shape, ii) a flattened polygonal shape, and iii) elongated/fusiform and spindle-like shapes. The distribution of different cell shapes changed with the distinct collagen fiber/fibril physical array and size. Dense collagen fibers, parallel to the culture plane, do not allow the invasion of MCF-7 and MDA-MB-231 cells, which, however, show increases of microvilli and microvesicles, respectively. These novel data highlight the regulatory role of different fibrillar collagen arrays in modifying breast cancer cell shape, inducing epithelial-to-mesenchymal transition, changing matrix composition and modulating the production of extracellular vesicles. Further investigation utilizing this in vitro model will help to demonstrate the biological roles of matrix macromolecules in cancer cell invasion in vivo.
Keywords: breast cancer; collagen type I; epithelial-to-mesenchymal transition; extracellular matrix; scanning electron microscope.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Garcia M.G., Bayo J., Bolontrade M.F., Sganga L., Malvicini M., Alaniz L., Aquino J.B., Fiore E., Rizzo M.M., Rodriguez A., et al. Hepatocellular carcinoma cells and their fibrotic microenvironment modulate bone marrow-derived mesenchymal stromal cell migration in vitro and in vivo. Mol. Pharm. 2011;8:1538–1548. doi: 10.1021/mp200137c. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

