Interactions between Host Immunity and Skin-Colonizing Staphylococci: No Two Siblings Are Alike
- PMID: 30736471
- PMCID: PMC6386899
- DOI: 10.3390/ijms20030718
Interactions between Host Immunity and Skin-Colonizing Staphylococci: No Two Siblings Are Alike
Abstract
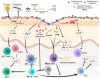
As the outermost layer of the body, the skin harbors innumerable and varied microorganisms. These microorganisms interact with the host, and these interactions contribute to host immunity. One of the most abundant genera of skin commensals is Staphylococcus. Bacteria belonging to this genus are some of the most influential commensals that reside on the skin. For example, colonization by Staphylococcus aureus, a well-known pathogen, increases inflammatory responses within the skin. Conversely, colonization by Staphylococcus epidermis, a coagulase-negative staphylococcal species that are prevalent throughout the skin, can be innocuous or beneficial. Thus, manipulating the abundance of these two bacterial species likely alters the skin microbiome and modulates the cutaneous immune response, with potential implications for various inflammation-associated skin diseases. Importantly, before researchers can begin manipulating the skin microbiome to prevent and treat disease, they must first fully understand how these two species can modulate the cutaneous immune response. In this review, we discuss the nature of the interactions between these two bacterial species and immune cells within the skin, discussing their immunogenicity within the context of skin disorders.
Keywords: Staphylococcus aureus; Staphylococcus epidermis; Staphylococcus spp., T cells; atopic dermatitis; commensals; cutaneous immunity; microbiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

