Inhibitory Effects of Antimicrobial Peptide JH-3 on Salmonella enterica Serovar Typhimurium Strain CVCC541 Infection-Induced Inflammatory Cytokine Release and Apoptosis in RAW264.7 Cells
- PMID: 30736473
- PMCID: PMC6384860
- DOI: 10.3390/molecules24030596
Inhibitory Effects of Antimicrobial Peptide JH-3 on Salmonella enterica Serovar Typhimurium Strain CVCC541 Infection-Induced Inflammatory Cytokine Release and Apoptosis in RAW264.7 Cells
Abstract
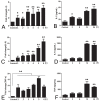
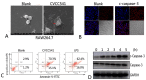
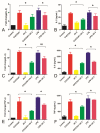
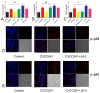
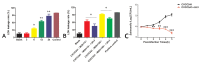
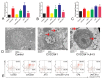
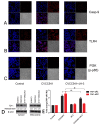
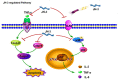
The antibiotic resistance of Salmonella has become increasingly serious due to the increased use of antibiotics, and antimicrobial peptides have been considered as an ideal antibiotic alternative. Salmonella can induce macrophage apoptosis and thus further damage the immune system. The antimicrobial peptide JH-3 has been shown to have a satisfactory anti-Salmonella effect in previous research, but its mechanism of action remains unknown. In this study, the effects of JH-3 on macrophages infected with Salmonella Typhimurium CVCC541 were evaluated at the cellular level. The results showed that JH-3 significantly alleviated the damage to macrophages caused by S. Typhi infection, reduced the release of lactic dehydrogenase (LDH), and killed the bacteria in macrophages. In addition, JH-3 decreased the phosphorylation level of p65 and the expression and secretion of interleukin 2 (IL-2), IL-6, and tumor necrosis factor-α (TNF-α) by inhibiting the activation of the mitogen-activated protein kinase (MAPK) (p38) signaling pathway and alleviating the cellular inflammatory response. From confocal laser scanning microscopy and flow cytometry assays, JH-3 was observed to inhibit the release of cytochrome c in the cytoplasm; the expression of TNF-αR2, caspase-9, and caspase-8; to further weaken caspase-3 activation; and to reduce the S.-Typhi-induced apoptosis of macrophages. In summary, the mechanism by which JH-3 inhibits Salmonella infection was systematically explored at the cellular level, laying the foundation for the development and utilization of JH-3 as a therapeutic alternative to antibiotics.
Keywords: Salmonella; antimicrobial peptide JH-3; apoptosis; cytokines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Majowicz S.E., Musto J., Scallan E., Angulo F.J., Kirk M., O’Brien S.J., Jones T.F., Fazil A., Hoekstra R.M. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. J. Food Saf. 2010;6:882–889. - PubMed
-
- Thomas M., Fenske G.J., Antony L., Ghimire S., Welsh R., Ramachandran A., Scaria J. Whole genome sequencing-based detection of antimicrobial resistance and virulence in non-typhoidal Salmonella enterica isolated from wildlife. Gut Pathog. 2017;1:66. doi: 10.1186/s13099-017-0213-x. - DOI - PMC - PubMed
-
- Bai L., Lan R., Zhang X., Cui S., Xu J., Guo Y., Li F., Zhang D. Prevalence of Salmonella Isolates from Chicken and Pig Slaughterhouses and Emergence of Ciprofloxacin and Cefotaxime Co-Resistant S. enterica Serovar Indiana in Henan, China. PLoS ONE. 2015;10:e014453212. doi: 10.1371/journal.pone.0144532. - DOI - PMC - PubMed
-
- El-Sharkawy H., Tahoun A., El-Gohary A.E.A., El-Abasy M., El-Khayat F., Gillespie T., Kitade Y., Hafez H.M., Neubauer H., El-Adawy H. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 2017;9:8. doi: 10.1186/s13099-017-0157-1. - DOI - PMC - PubMed
-
- Flores- Alvarez L.J., Guzmán- Rodríguez J.J., López- Gómez R., Salgado- Garciglia R., Ochoa- Zarzosa A., López- Meza J.E. PaDef defensin from avocado (Persea americana var. drymifolia) is cytotoxic to K562 chronic myeloid leukemia cells through extrinsic apoptosis. Int. J. Biochem. Cell Biol. 2018;99:10–18. doi: 10.1016/j.biocel.2018.03.013. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 31702259 and 31672559/National Natural Science Foundation of China/International
- 2016YFD0500708-04/National Key Research and Development Program of China/International
- 174100510005/the Excellent Youth Foundation of Henan Scientific Committee/International
- 14HASTIT026/the Program for Science Technology Innovation Talents in Universities of Henan Province/International
LinkOut - more resources
Full Text Sources
Research Materials

