Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer
- PMID: 30736848
- PMCID: PMC6368815
- DOI: 10.1186/s40425-019-0520-5
Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer
Abstract
Background: Preclinical studies demonstrate synergism between cancer immunotherapy and local radiation, enhancing anti-tumor effects and promoting immune responses. BI1361849 (CV9202) is an active cancer immunotherapeutic comprising protamine-formulated, sequence-optimized mRNA encoding six non-small cell lung cancer (NSCLC)-associated antigens (NY-ESO-1, MAGE-C1, MAGE-C2, survivin, 5T4, and MUC-1), intended to induce targeted immune responses.
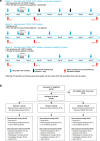
Methods: We describe a phase Ib clinical trial evaluating treatment with BI1361849 combined with local radiation in 26 stage IV NSCLC patients with partial response (PR)/stable disease (SD) after standard first-line therapy. Patients were stratified into three strata (1: non-squamous NSCLC, no epidermal growth factor receptor (EGFR) mutation, PR/SD after ≥4 cycles of platinum- and pemetrexed-based treatment [n = 16]; 2: squamous NSCLC, PR/SD after ≥4 cycles of platinum-based and non-platinum compound treatment [n = 8]; 3: non-squamous NSCLC, EGFR mutation, PR/SD after ≥3 and ≤ 6 months EGFR-tyrosine kinase inhibitor (TKI) treatment [n = 2]). Patients received intradermal BI1361849, local radiation (4 × 5 Gy), then BI1361849 until disease progression. Strata 1 and 3 also had maintenance pemetrexed or continued EGFR-TKI therapy, respectively. The primary endpoint was evaluation of safety; secondary objectives included assessment of clinical efficacy (every 6 weeks during treatment) and of immune response (on Days 1 [baseline], 19 and 61).
Results: Study treatment was well tolerated; injection site reactions and flu-like symptoms were the most common BI1361849-related adverse events. Three patients had grade 3 BI1361849-related adverse events (fatigue, pyrexia); there was one grade 3 radiation-related event (dysphagia). In comparison to baseline, immunomonitoring revealed increased BI1361849 antigen-specific immune responses in the majority of patients (84%), whereby antigen-specific antibody levels were increased in 80% and functional T cells in 40% of patients, and involvement of multiple antigen specificities was evident in 52% of patients. One patient had a partial response in combination with pemetrexed maintenance, and 46.2% achieved stable disease as best overall response. Best overall response was SD in 57.7% for target lesions.
Conclusion: The results support further investigation of mRNA-based immunotherapy in NSCLC including combinations with immune checkpoint inhibitors.
Trial registration: ClinicalTrials.gov identifier: NCT01915524 .
Keywords: BI1361849; CV9202; Clinical trial; Hypofractionated radiotherapy; Immunomonitoring; Non-small cell lung cancer; mRNA active cancer immunotherapy.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by local ethics committees. The names and reference numbers are provided in Additional file 11: Table S7.
All patients provided written informed consent before receiving any study-related procedures.
Consent for publication
Not applicable
Competing interests
AP declares non-financial support from CureVac during the conduct of the study. MS declares personal fees from Boehringer Ingelheim, BMS, MSD, Lilly, Pfizer, Astra Zeneca, Roche, Celgen, and Novartis and non-financial support from Boehringer Ingelheim, BMS, and Novartis outside the submitted work. MP declares personal fees from Astra Zeneca, BMS, Boehringer Ingelheim, Merck, Novartis, Pfizer, and Roche outside the submitted work. FG declares grants and personal fees from Astra Zeneca, Boehringer Ingelheim, Pfizer, Lilly, BMS, MSD, Novartis, Celgene, and Roche and personal fees from Ariad, Takeda, and Chugai outside the submitted work. AZ declares study fees from CureVac during the conduct of the study and research funding from BeyondSprings, Secarna, and Roche outside the submitted work. UK, MFM, BS, CS, AM, TS, FD and UG-V are employees of CureVac. AS, MMH, HSH and SDK were employees of CureVac during the conduct of this study. K-JK was a consultant to CureVac until 2015 and was an employee at CureVac until 2012. K-JK, MFM and UG-V jointly hold patent WO/2015/024666. MF, CW, RC, WH, GP, TW, JA, HB, and MG have no conflicts of interest to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68. doi: 10.1016/S1470-2045(13)70510-2. - DOI - PubMed
-
- Vansteenkiste JF, Cho BC, Vanakesa T, De Pas T, Zielinski M, Kim MS, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:822–835. doi: 10.1016/S1470-2045(16)00099-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
