The early identification of disease progression in patients with suspected infection presenting to the emergency department: a multi-centre derivation and validation study
- PMID: 30736862
- PMCID: PMC6368690
- DOI: 10.1186/s13054-019-2329-5
The early identification of disease progression in patients with suspected infection presenting to the emergency department: a multi-centre derivation and validation study
Erratum in
-
Correction to: The early identification of disease progression in patients with suspected infection presenting to the emergency department: a multi-centre derivation and validation study.Crit Care. 2019 Jul 15;23(1):255. doi: 10.1186/s13054-019-2536-0. Crit Care. 2019. PMID: 31307526 Free PMC article.
Abstract
Background: There is a lack of validated tools to assess potential disease progression and hospitalisation decisions in patients presenting to the emergency department (ED) with a suspected infection. This study aimed to identify suitable blood biomarkers (MR-proADM, PCT, lactate and CRP) or clinical scores (SIRS, SOFA, qSOFA, NEWS and CRB-65) to fulfil this unmet clinical need.
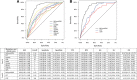
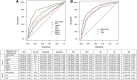
Methods: An observational derivation patient cohort validated by an independent secondary analysis across nine EDs. Logistic and Cox regression, area under the receiver operating characteristic (AUROC) and Kaplan-Meier curves were used to assess performance. Disease progression was identified using a composite endpoint of 28-day mortality, ICU admission and hospitalisation > 10 days.
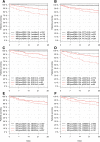
Results: One thousand one hundred seventy-five derivation and 896 validation patients were analysed with respective 28-day mortality rates of 7.1% and 5.0%, and hospitalisation rates of 77.9% and 76.2%. MR-proADM showed greatest accuracy in predicting 28-day mortality and hospitalisation requirement across both cohorts. Patient subgroups with high MR-proADM concentrations (≥ 1.54 nmol/L) and low biomarker (PCT < 0.25 ng/mL, lactate < 2.0 mmol/L or CRP < 67 mg/L) or clinical score (SOFA < 2 points, qSOFA < 2 points, NEWS < 4 points or CRB-65 < 2 points) values were characterised by a significantly longer length of hospitalisation (p < 0.001), rate of ICU admission (p < 0.001), elevated mortality risk (e.g. SOFA, qSOFA and NEWS HR [95%CI], 45.5 [10.0-207.6], 23.4 [11.1-49.3] and 32.6 [9.4-113.6], respectively) and a greater number of disease progression events (p < 0.001), compared to similar subgroups with low MR-proADM concentrations (< 1.54 nmol/L). Increased out-patient treatment across both cohorts could be facilitated using a derivation-derived MR-proADM cut-off of < 0.87 nmol/L (15.0% and 16.6%), with decreased readmission rates and no mortalities.
Conclusions: In patients presenting to the ED with a suspected infection, the blood biomarker MR-proADM could most accurately identify the likelihood of further disease progression. Incorporation into an early sepsis management protocol may therefore aid rapid decision-making in order to either initiate, escalate or intensify early treatment strategies, or identify patients suitable for safe out-patient treatment.
Keywords: Disease progression; Emergency department; MR-proADM; SOFA; Sepsis; qSOFA.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the ethics board of each hospital where necessary, and written informed consent was obtained from all patients or their legal representatives where appropriate.
Consent for publication
No individual participant data is reported that would require consent to publish from the participant (or legal parent or guardian for children).
Competing interests
All authors have provided information on potential conflicts of interests directly or indirectly related to the work submitted in the journal’s disclosure forms. KS received research grant paid to the insitituion from Thermofisher, as well as educational grants and support to attend meetings. PS and BM received research support paid to the Institution from bioMerieux and Thermofisher. AK received support from BRAHMS to attend meetings and fulfilled speaking engagements. PH received Lecture honorarium (ThermoFisher Scientific, Beckman Coulter), Educational support honorarium (bioMérieux) Clinical research grants (ThermoFisher Scientific, bioMérieux). DCW is an employee of BRAHMS GmbH, which holds patent rights on the procalcitonin and mid-regional proadrenomedullin assay. All other authors reported no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Comment in
-
Application of MR-ProADM to predict prevention of hospitalisation, derived from a multi-centre study.Crit Care. 2019 Apr 17;23(1):129. doi: 10.1186/s13054-019-2402-0. Crit Care. 2019. PMID: 30995933 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

