Fibrinogen Induces Microglia-Mediated Spine Elimination and Cognitive Impairment in an Alzheimer's Disease Model
- PMID: 30737131
- PMCID: PMC6602536
- DOI: 10.1016/j.neuron.2019.01.014
Fibrinogen Induces Microglia-Mediated Spine Elimination and Cognitive Impairment in an Alzheimer's Disease Model
Abstract
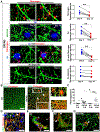
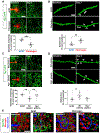
Cerebrovascular alterations are a key feature of Alzheimer's disease (AD) pathogenesis. However, whether vascular damage contributes to synaptic dysfunction and how it synergizes with amyloid pathology to cause neuroinflammation and cognitive decline remain poorly understood. Here, we show that the blood protein fibrinogen induces spine elimination and promotes cognitive deficits mediated by CD11b-CD18 microglia activation. 3D molecular labeling in cleared mouse and human AD brains combined with repetitive in vivo two-photon imaging showed focal fibrinogen deposits associated with loss of dendritic spines independent of amyloid plaques. Fibrinogen-induced spine elimination was prevented by inhibiting reactive oxygen species (ROS) generation or genetic ablation of CD11b. Genetic elimination of the fibrinogen binding motif to CD11b reduced neuroinflammation, synaptic deficits, and cognitive decline in the 5XFAD mouse model of AD. Thus, fibrinogen-induced spine elimination and cognitive decline via CD11b link cerebrovascular damage with immune-mediated neurodegeneration and may have important implications in AD and related conditions.
Keywords: blood-brain barrier; coagulation; complement; dementia; dendritic spines; fibrin; iDISCO; innate immunity; multiple sclerosis; neurovascular.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
H. Lundbeck A/S sponsored research in K.A.'s laboratory at the Gladstone Institutes. K.A. is a founder of MedaRed, Inc. Her interests are managed by the Gladstone Institutes in accordance with its conflict-of-interest policy. R.B.N. was an employee of Lundbeck during the time this work was performed.
Figures


Comment in
-
Fibrinogen links vascular pathology to cognitive decline.Nat Rev Neurol. 2019 Apr;15(4):187. doi: 10.1038/s41582-019-0154-8. Nat Rev Neurol. 2019. PMID: 30783220 No abstract available.
-
Inflaming the Brain.Neuron. 2019 Mar 20;101(6):991-993. doi: 10.1016/j.neuron.2019.03.007. Neuron. 2019. PMID: 30897363
References
-
- Bien-Ly N, Boswell CA, Jeet S, Beach TG, Hoyte K, Luk W, Shihadeh V, Ulufatu S, Foreman O, Lu Y, et al. (2015). Lack of widespread BBB disruption in Alzheimer's disease models: Focus on therapeutic antibodies. Neuron 88, 289–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

