Biallelic variants in LARS2 and KARS cause deafness and (ovario)leukodystrophy
- PMID: 30737337
- PMCID: PMC9281382
- DOI: 10.1212/WNL.0000000000007098
Biallelic variants in LARS2 and KARS cause deafness and (ovario)leukodystrophy
Erratum in
-
Biallelic variants in LARS2 and KARS cause deafness and (ovario)leukodystrophy.Neurology. 2019 Nov 26;93(22):982. doi: 10.1212/WNL.0000000000007422. Neurology. 2019. PMID: 31767665 Free PMC article. No abstract available.
Abstract
Objective: To describe the leukodystrophy caused by pathogenic variants in LARS2 and KARS, encoding mitochondrial leucyl transfer RNA (tRNA) synthase and mitochondrial and cytoplasmic lysyl tRNA synthase, respectively.
Methods: We composed a group of 5 patients with leukodystrophy, in whom whole-genome or whole-exome sequencing revealed pathogenic variants in LARS2 or KARS. Clinical information, brain MRIs, and postmortem brain autopsy data were collected. We assessed aminoacylation activities of purified mutant recombinant mitochondrial leucyl tRNA synthase and performed aminoacylation assays on patients' lymphoblasts and fibroblasts.
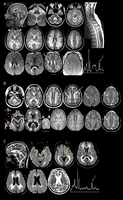
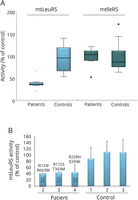
Results: Patients had a combination of early-onset deafness and later-onset neurologic deterioration caused by progressive brain white matter abnormalities on MRI. Female patients with LARS2 pathogenic variants had premature ovarian failure. In 2 patients, MRI showed additional signs of early-onset vascular abnormalities. In 2 other patients with LARS2 and KARS pathogenic variants, magnetic resonance spectroscopy revealed elevated white matter lactate, suggesting mitochondrial disease. Pathology in one patient with LARS2 pathogenic variants displayed evidence of primary disease of oligodendrocytes and astrocytes with lack of myelin and deficient astrogliosis. Aminoacylation activities of purified recombinant mutant leucyl tRNA synthase showed a 3-fold loss of catalytic efficiency. Aminoacylation assays on patients' lymphoblasts and fibroblasts showed about 50% reduction of enzyme activity.
Conclusion: This study adds LARS2 and KARS pathogenic variants as gene defects that may underlie deafness, ovarian failure, and leukodystrophy with mitochondrial signature. We discuss the specific MRI characteristics shared by leukodystrophies caused by mitochondrial tRNA synthase defects. We propose to add aminoacylation assays as biochemical diagnostic tools for leukodystrophies.
© 2019 American Academy of Neurology.
Figures



References
-
- Kevelam SH, Steenweg ME, Srivastava S, et al. Update on leukodystrophies: a historical perspective and adapted definition. Neuropediatrics 2016;47:349–354. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
