Lithium promoted mesoporous manganese oxide catalyzed oxidation of allyl ethers
- PMID: 30737383
- PMCID: PMC6368603
- DOI: 10.1038/s41467-019-08619-x
Lithium promoted mesoporous manganese oxide catalyzed oxidation of allyl ethers
Abstract
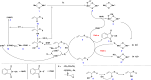
Herein we report the first example of the catalytic aerobic partial oxidation of allyl ether to its acrylate ester derivative. Many partial oxidations often need an expensive oxidant such as peroxides or other species to drive such reactions. In addition, selective generation of esters using porous catalysts has been elusive. This reaction is catalyzed by a Li ion promoted mesoporous manganese oxide (meso-Mn2O3) under mild conditions with no precious metals, a reusable heterogeneous catalyst, and easy isolation. This process is very attractive for the oxidation of allyl ethers. We report on the catalytic activity, selectivity, and scope of the reaction. In the best cases presented, almost complete conversion of allyl ether with near complete chemo-selectivity towards acrylate ester derivatives is observed. Based on results from controlled experiments, we propose a possible reaction mechanism for the case in which N-hydroxyphthalimide (NHPI) is used in combination with trichloroacetonitrile (CCl3CN).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Zhang Z, Qu Y, Wang S, Wang J. Catalytic performance and characterization of silica supported sodium phosphates for the dehydration of methyl lactate to methyl acrylate andacrylic acid. Ind. Eng. Chem. Res. 2009;48:9083–9089. doi: 10.1021/ie900065a. - DOI
-
- Lee JM, et al. Efficient dehydration of methyl lactate to acrylic acid using Ca 3(PO4)2-SiO2 catalyst. Catal. Commun. 2010;11:1176–1180. doi: 10.1016/j.catcom.2010.06.013. - DOI
-
- Zhang J, et al. Efficient acrylic acid production through bio lactic acid dehydration over NaY Zeolite modified by alkali phosphates. ACS Catal. 2011;1:32–41. doi: 10.1021/cs100047p. - DOI
-
- Jamróz MH, Jamróz ME, Rode JE, Bednarek E, Dobrowolski JC. Interpretation of vibrational and NMR spectra of allyl acrylate: An evidence for several conformers. Vib. Spectrosc. 2009;50:231–244. doi: 10.1016/j.vibspec.2009.01.001. - DOI
-
- Agarwal YK, Kaushik SD, Kumar PC. Synthesis and rheological studies of methacrylic acid‐ethyl acrylate–allyl methacrylate terpolymers. J. Macromol. Sci. Part A. 2007;44:877–880. doi: 10.1080/10601320701407896. - DOI
Publication types
LinkOut - more resources
Full Text Sources

