Ionic current correlations are ubiquitous across phyla
- PMID: 30737430
- PMCID: PMC6368568
- DOI: 10.1038/s41598-018-38405-6
Ionic current correlations are ubiquitous across phyla
Abstract
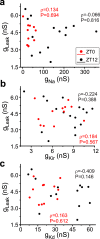
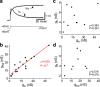
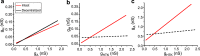
Ionic currents, whether measured as conductance amplitude or as ion channel transcript numbers, can vary many-fold within a population of identified neurons. In invertebrate neuronal types multiple currents can be seen to vary while at the same time their magnitudes are correlated. These conductance amplitude correlations are thought to reflect a tight homeostasis of cellular excitability that enhances the robustness and stability of neuronal activity over long stretches of time. Although such ionic conductance correlations are well documented in invertebrates, they have not been reported in vertebrates. Here we demonstrate with two examples, identified mouse hippocampal granule cells (GCs) and cholinergic basal forebrain neurons, that the correlation of ionic conductance amplitudes between different ionic currents also exists in vertebrates, and we argue that it is a ubiquitous phenomenon expressed by many species across phyla. We further demonstrate that in dentate gyrus GCs these conductance correlations are likely regulated in a circadian manner. This is reminiscent of the known conductance regulation by neuromodulators in crustaceans. However, in GCs we observe a more nuanced regulation, where for some conductance pairs the correlations are completely eliminated while for others the correlation is quantitatively modified but not obliterated.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

