Regulation of the innate immune system by autophagy: neutrophils, eosinophils, mast cells, NK cells
- PMID: 30737478
- PMCID: PMC6460399
- DOI: 10.1038/s41418-019-0295-8
Regulation of the innate immune system by autophagy: neutrophils, eosinophils, mast cells, NK cells
Abstract
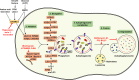
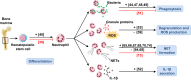
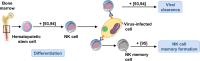
Autophagy is an evolutionally conserved, highly regulated catabolic process that combines cellular functions required for the regulation of metabolic balance under conditions of stress with those needed for the degradation of damaged cell organelles via the lysosomal machinery. The importance of autophagy for cell homeostasis and survival has long been appreciated. Recent data suggest that autophagy is also involved in non-metabolic functions that impact the immune system. Here, we reflect in two review articles the recent literature pointing to an important role for autophagy in innate immune cells. In this article, we focus on neutrophils, eosinophils, mast cells, and natural killer cells. We mainly discuss the influence of autophagy on functional cellular responses and its importance for overall host defense. In the companion review, we present the role of autophagy in the functions performed by monocytes/macrophages and dendritic cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

