ADEpedia-on-OHDSI: A next generation pharmacovigilance signal detection platform using the OHDSI common data model
- PMID: 30738946
- PMCID: PMC6432939
- DOI: 10.1016/j.jbi.2019.103119
ADEpedia-on-OHDSI: A next generation pharmacovigilance signal detection platform using the OHDSI common data model
Abstract
Objective: Supplementing the Spontaneous Reporting System (SRS) with Electronic Health Record (EHR) data for adverse drug reaction detection could augment sample size, increase population heterogeneity and cross-validate results for pharmacovigilance research. The difference in the underlying data structures and terminologies between SRS and EHR data presents challenges when attempting to integrate the two into a single database. The Observational Health Data Sciences and Informatics (OHDSI) collaboration provides a Common Data Model (CDM) for organizing and standardizing EHR data to support large-scale observational studies. The objective of the study is to develop and evaluate an informatics platform known as ADEpedia-on-OHDSI, where spontaneous reporting data from FDA's Adverse Event Reporting System (FAERS) is converted into the OHDSI CDM format towards building a next generation pharmacovigilance signal detection platform.
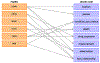
Methods: An extraction, transformation and loading (ETL) tool was designed, developed, and implemented to convert FAERS data into the OHDSI CDM format. A comprehensive evaluation, including overall ETL evaluation, mapping quality evaluation of drug names to RxNorm, and an evaluation of transformation and imputation quality, was then performed to assess the mapping accuracy and information loss using the FAERS data collected between 2012 and 2017. Previously published findings related to vascular safety profile of triptans were validated using ADEpedia-on-OHDSI in pharmacovigilance research. For the triptan-related vascular event detection, signals were detected by Reporting Odds Ratio (ROR) in high-level group terms (HLGT) level, high-level terms (HLT) level and preferred term (PT) level using the original FAERS data and CDM-based FAERS respectively. In addition, six standardized MedDRA queries (SMQs) related to vascular events were applied.
Results: A total of 4,619,362 adverse event cases were loaded into 8 tables in the OHDSI CDM. For drug name mapping, 93.9% records and 47.0% unique names were matched with RxNorm codes. Mapping accuracy of drug names was 96% based on a manual verification of randomly sampled 500 unique mappings. Information loss evaluation showed that more than 93% of the data is loaded into the OHDSI CDM for most fields, with the exception of drug route data (66%). The replication study detected 5, 18, 47 and 6, 18, 50 triptan-related vascular event signals in MedDRA HLGT level, HLT level, and PT level for the original FAERS data and CDM-based FAERS respectively. The signal detection scores of six standardized MedDRA queries (SMQs) of vascular events in the raw data study were found to be lower than those scores in the CDM study.
Conclusion: The outcome of this work would facilitate seamless integration and combined analyses of both SRS and EHR data for pharmacovigilance in ADEpedia-on-OHDSI, our platform for next generation pharmacovigilance.
Keywords: Data Standardization; ETL tool; FAERS; OHDSI Common Data Model; Pharmacovigilance.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

