The Structural Model: a theory linking connections, plasticity, pathology, development and evolution of the cerebral cortex
- PMID: 30739157
- PMCID: PMC6500485
- DOI: 10.1007/s00429-019-01841-9
The Structural Model: a theory linking connections, plasticity, pathology, development and evolution of the cerebral cortex
Abstract
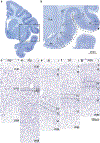
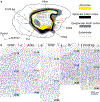
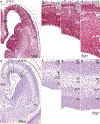
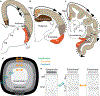
The classical theory of cortical systematic variation has been independently described in reptiles, monotremes, marsupials and placental mammals, including primates, suggesting a common bauplan in the evolution of the cortex. The Structural Model is based on the systematic variation of the cortex and is a platform for advancing testable hypotheses about cortical organization and function across species, including humans. The Structural Model captures the overall laminar structure of areas by dividing the cortical architectonic continuum into discrete categories (cortical types), which can be used to test hypotheses about cortical organization. By type, the phylogenetically ancient limbic cortices-which form a ring at the base of the cerebral hemisphere-are agranular if they lack layer IV, or dysgranular if they have an incipient granular layer IV. Beyond the dysgranular areas, eulaminate type cortices have six layers. The number and laminar elaboration of eulaminate areas differ depending on species or cortical system within a species. The construct of cortical type retains the topology of the systematic variation of the cortex and forms the basis for a predictive Structural Model, which has successfully linked cortical variation to the laminar pattern and strength of cortical connections, the continuum of plasticity and stability of areas, the regularities in the distribution of classical and novel markers, and the preferential vulnerability of limbic areas to neurodegenerative and psychiatric diseases. The origin of cortical types has been recently traced to cortical development, and helps explain the variability of diseases with an onset in ontogeny.
Keywords: Brain pathology; Cortical hierarchies; Glia; Homology; Limbic cortex; Phylogeny.
Conflict of interest statement
Figures





References
-
- Abbie AA (1940) Cortical lamination in the monotremata. J Comp Neurol 72:429–467
-
- Abbie AA (1942) Cortical lamination in a polyprotodont marsupial, perameles nasuta. J Comp Neurol 76:509–536
-
- Allman J (2000) Evolving brains Scientific American Library, New York
-
- Ariëns Kappers CU, Huber GC, Crosby EC (1936) The comparative anatomy of the nervous system of vertebrates, including man Macmillan, New York,
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW (1991) The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex 1:103–116 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

