Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials
- PMID: 30739743
- PMCID: PMC6451189
- DOI: 10.1016/S0140-6736(18)33137-4
Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials
Abstract
Background: Increasing the dose intensity of cytotoxic therapy by shortening the intervals between cycles, or by giving individual drugs sequentially at full dose rather than in lower-dose concurrent treatment schedules, might enhance efficacy.
Methods: To clarify the relative benefits and risks of dose-intense and standard-schedule chemotherapy in early breast cancer, we did an individual patient-level meta-analysis of trials comparing 2-weekly versus standard 3-weekly schedules, and of trials comparing sequential versus concurrent administration of anthracycline and taxane chemotherapy. The primary outcomes were recurrence and breast cancer mortality. Standard intention-to-treat log-rank analyses, stratified by age, nodal status, and trial, yielded dose-intense versus standard-schedule first-event rate ratios (RRs).
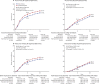
Findings: Individual patient data were provided for 26 of 33 relevant trials identified, comprising 37 298 (93%) of 40 070 women randomised. Most women were aged younger than 70 years and had node-positive disease. Total cytotoxic drug usage was broadly comparable in the two treatment arms; colony-stimulating factor was generally used in the more dose-intense arm. Combining data from all 26 trials, fewer breast cancer recurrences were seen with dose-intense than with standard-schedule chemotherapy (10-year recurrence risk 28·0% vs 31·4%; RR 0·86, 95% CI 0·82-0·89; p<0·0001). 10-year breast cancer mortality was similarly reduced (18·9% vs 21·3%; RR 0·87, 95% CI 0·83-0·92; p<0·0001), as was all-cause mortality (22·1% vs 24·8%; RR 0·87, 95% CI 0·83-0·91; p<0·0001). Death without recurrence was, if anything, lower with dose-intense than with standard-schedule chemotherapy (10-year risk 4·1% vs 4·6%; RR 0·88, 95% CI 0·78-0·99; p=0·034). Recurrence reductions were similar in the seven trials (n=10 004) that compared 2-weekly chemotherapy with the same chemotherapy given 3-weekly (10-year risk 24·0% vs 28·3%; RR 0·83, 95% CI 0·76-0·91; p<0·0001), in the six trials (n=11 028) of sequential versus concurrent anthracycline plus taxane chemotherapy (28·1% vs 31·3%; RR 0·87, 95% CI 0·80-0·94; p=0·0006), and in the six trials (n=6532) testing both shorter intervals and sequential administration (30·4% vs 35·0%; RR 0·82, 95% CI 0·74-0·90; p<0·0001). The proportional reductions in recurrence with dose-intense chemotherapy were similar and highly significant (p<0·0001) in oestrogen receptor (ER)-positive and ER-negative disease and did not differ significantly by other patient or tumour characteristics.
Interpretation: Increasing the dose intensity of adjuvant chemotherapy by shortening the interval between treatment cycles, or by giving individual drugs sequentially rather than giving the same drugs concurrently, moderately reduces the 10-year risk of recurrence and death from breast cancer without increasing mortality from other causes.
Funding: Cancer Research UK, Medical Research Council.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures





Comment in
-
Improving chemotherapy outcome in early breast cancer.Gland Surg. 2019 Oct;8(5):585-587. doi: 10.21037/gs.2019.08.04. Gland Surg. 2019. PMID: 31741891 Free PMC article. No abstract available.
-
Shorter intervals and sequential administration of adjuvant chemotherapy are effective in reducing the 10-year risk of recurrence and death in early breast cancer women without detrimental effects.Evid Based Nurs. 2021 Apr;24(2):62-63. doi: 10.1136/ebnurs-2019-103138. Epub 2020 Mar 3. Evid Based Nurs. 2021. PMID: 32132126 No abstract available.
References
-
- Earl HM, Hiller L, Howard HC. Addition of gemcitabine to paclitaxel, epirubicin, and cyclophosphamide adjuvant chemotherapy for women with early-stage breast cancer (tAnGo): final 10-year follow-up of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:755–769. - PubMed
-
- Hryniuk WM. Average relative dose intensity and the impact on design of clinical trials. Semin Oncol. 1997;14:65–74. - PubMed

