Tumour cell blebbing and extracellular vesicle shedding: key role of matrikines and ribosomal protein SA
- PMID: 30739912
- PMCID: PMC6461924
- DOI: 10.1038/s41416-019-0382-0
Tumour cell blebbing and extracellular vesicle shedding: key role of matrikines and ribosomal protein SA
Abstract
Background: Carcinogenesis occurs in elastin-rich tissues and leads to local inflammation and elastolytic proteinase release. This contributes to bioactive matrix fragment (Matrikine) accumulation like elastin degradation products (EDP) stimulating tumour cell invasive and metastatic properties. We previously demonstrate that EDPs exert protumoural activities through Hsp90 secretion to stabilised extracellular proteinases.
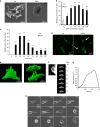
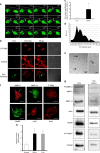
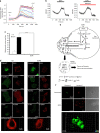
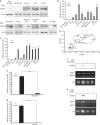
Methods: EDP influence on cancer cell blebbing and extracellular vesicle shedding were examined with a videomicroscope coupled with confocal Yokogawa spinning disk, by transmission electron microscopy, scanning electron microscopy and confocal microscopy. The ribosomal protein SA (RPSA) elastin receptor was identified after affinity chromatography by western blotting and cell immunolocalisation. mRNA expression was studied using real-time PCR. SiRNA were used to confirm the essential role of RPSA.
Results: We demonstrate that extracellular matrix degradation products like EDPs induce tumour amoeboid phenotype with cell membrane blebbing and shedding of extracellular vesicle containing Hsp90 and proteinases in the extracellular space. EDPs influence intracellular calcium influx and cytoskeleton reorganisation. Among matrikines, VGVAPG and AGVPGLGVG peptides reproduced EDP effects through RPSA binding.
Conclusions: Our data suggests that matrikines induce cancer cell blebbing and extracellular vesicle release through RPSA binding, favouring dissemination, cell-to-cell communication and growth of cancer cells in metastatic sites.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Elastin peptides regulate HT-1080 fibrosarcoma cell migration and invasion through an Hsp90-dependent mechanism.Br J Cancer. 2014 Jul 8;111(1):139-48. doi: 10.1038/bjc.2014.239. Epub 2014 May 29. Br J Cancer. 2014. PMID: 24874477 Free PMC article.
-
A multimodal imaging study to highlight elastin-derived peptide pro-tumoral effect in a pancreatic xenograft model.Br J Cancer. 2023 Jun;128(11):2000-2012. doi: 10.1038/s41416-023-02242-w. Epub 2023 Mar 31. Br J Cancer. 2023. PMID: 37002342 Free PMC article.
-
Matrikines for therapeutic and biomedical applications.Life Sci. 2018 Dec 1;214:22-33. doi: 10.1016/j.lfs.2018.10.056. Epub 2018 Oct 26. Life Sci. 2018. PMID: 30449450 Review.
-
AG-9, an Elastin-Derived Peptide, Increases In Vitro Oral Tongue Carcinoma Cell Invasion, through an Increase in MMP-2 Secretion and MT1-MMP Expression, in a RPSA-Dependent Manner.Biomolecules. 2020 Dec 30;11(1):39. doi: 10.3390/biom11010039. Biomolecules. 2020. PMID: 33396696 Free PMC article.
-
Elastin as a matrikine.Crit Rev Oncol Hematol. 2004 Mar;49(3):235-44. doi: 10.1016/j.critrevonc.2003.09.007. Crit Rev Oncol Hematol. 2004. PMID: 15036263 Review.
Cited by
-
Dysregulation of intercellular communication in vitro and in vivo via extracellular vesicles secreted by pancreatic duct adenocarcinoma cells and generated under the influence of the AG9 elastin peptide-conditioned microenvironment.J Extracell Biol. 2024 Mar 7;3(3):e145. doi: 10.1002/jex2.145. eCollection 2024 Mar. J Extracell Biol. 2024. PMID: 38939412 Free PMC article.
-
Endothelial Basement Membrane Components and Their Products, Matrikines: Active Drivers of Pulmonary Hypertension?Cells. 2020 Sep 3;9(9):2029. doi: 10.3390/cells9092029. Cells. 2020. PMID: 32899187 Free PMC article. Review.
-
Beyond Blood Clotting: The Many Roles of Platelet-Derived Extracellular Vesicles.Biomedicines. 2024 Aug 14;12(8):1850. doi: 10.3390/biomedicines12081850. Biomedicines. 2024. PMID: 39200314 Free PMC article. Review.
-
Distinct roles of protrusions and collagen deformation in collective invasion of cancer cell types.Biophys J. 2025 May 6;124(9):1506-1520. doi: 10.1016/j.bpj.2025.03.032. Epub 2025 Mar 31. Biophys J. 2025. PMID: 40170350 Free PMC article.
-
Urinary extracellular vesicles miRNA-A new era of prostate cancer biomarkers.Front Genet. 2023 Jan 20;14:1065757. doi: 10.3389/fgene.2023.1065757. eCollection 2023. Front Genet. 2023. PMID: 36741322 Free PMC article. Review.
References
-
- Rowe, R. G., Weiss, S. J. Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu Rev. Cell Dev. Biol. 25, 567–595 (2009). - PubMed
-
- Maquart, F. X., Pasco, S., Ramont, L., Hornebeck, W. & Monboisse, J. C. An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity: implication in tumor invasion. Crit. Rev. Oncol. Hematol.49, 199–202 (2004). - PubMed
-
- Brassart B, Randoux A, Hornebeck W, Emonard H. Regulation of matrix metalloproteinase-2 (gelatinase A, MMP-2), membrane-type matrix metalloproteinase-1 (MT1-MMP) and tissue inhibitor of metalloproteinases-2 (TIMP-2) expression by elastin-derived peptides in human HT-1080 fibrosarcoma cell line. Clin. Exp. Metastas-. 1998;16:489–500. doi: 10.1023/A:1006550503612. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

