Nanoscale Metal-Organic Frameworks for Phototherapy of Cancer
- PMID: 30739946
- PMCID: PMC6366651
- DOI: 10.1016/j.ccr.2017.09.007
Nanoscale Metal-Organic Frameworks for Phototherapy of Cancer
Abstract
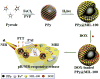
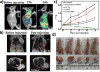
Phototherapy involves the irradiation of tissues with light, and is commonly implemented in the forms of photodynamic therapy (PDT) and photothermal therapy (PTT). Photosensitizers (PSs) are often needed to improve the efficacy and selectivity of phototherapy via enhanced singlet oxygen generation in PDT and photothermal responses in PTT. In both cases, efficient and selective delivery of PSs to the diseased tissues is of paramount importance. Nanoscale metal-organic frameworks (nMOFs), a new class of hybrid materials built from metal connecting points and bridging ligands, have been examined as nanocarriers for drug delivery due to their compositional and structural tunability, highly porous structures, and good biocompatibility. This review summarizes recent advances on using nMOFs as nanoparticle PSs for applications in PDT and PTT.
Keywords: Metal-organic framework; Nanophotosensitizers; Photodynamic therapy; Photothermal therapy.
Figures




Similar articles
-
Nanoscale Metal-Organic Frameworks for Cancer Immunotherapy.Acc Chem Res. 2020 Sep 15;53(9):1739-1748. doi: 10.1021/acs.accounts.0c00313. Epub 2020 Aug 18. Acc Chem Res. 2020. PMID: 32808760 Free PMC article.
-
Nanoscale metal-organic frameworks as photosensitizers and nanocarriers in photodynamic therapy.Front Chem. 2022 Aug 26;10:971747. doi: 10.3389/fchem.2022.971747. eCollection 2022. Front Chem. 2022. PMID: 36092660 Free PMC article. Review.
-
Recent advances in the nanoarchitectonics of metal-organic frameworks for light-activated tumor therapy.Dalton Trans. 2023 Nov 14;52(44):16085-16102. doi: 10.1039/d3dt02725b. Dalton Trans. 2023. PMID: 37814810 Review.
-
Photodynamic Therapy Based on Nanoscale Metal-Organic Frameworks: From Material Design to Cancer Nanotherapeutics.Chem Asian J. 2018 Nov 2;13(21):3122-3149. doi: 10.1002/asia.201801221. Epub 2018 Oct 12. Chem Asian J. 2018. PMID: 30183134 Review.
-
Nanoscale Covalent Organic Framework for Combinatorial Antitumor Photodynamic and Photothermal Therapy.ACS Nano. 2019 Nov 26;13(11):13304-13316. doi: 10.1021/acsnano.9b06467. Epub 2019 Nov 8. ACS Nano. 2019. PMID: 31689082
Cited by
-
Heterostructures Made of Upconversion Nanoparticles and Metal-Organic Frameworks for Biomedical Applications.Adv Sci (Weinh). 2022 Jan;9(3):e2103911. doi: 10.1002/advs.202103911. Epub 2021 Nov 17. Adv Sci (Weinh). 2022. PMID: 34791801 Free PMC article. Review.
-
Microneedle-Mediated Treatment of Obesity.Pharmaceutics. 2025 Feb 13;17(2):248. doi: 10.3390/pharmaceutics17020248. Pharmaceutics. 2025. PMID: 40006614 Free PMC article. Review.
-
A Human Feedback Strategy for Photoresponsive Molecules in Drug Delivery: Utilizing GPT-2 and Time-Dependent Density Functional Theory Calculations.Pharmaceutics. 2024 Jul 31;16(8):1014. doi: 10.3390/pharmaceutics16081014. Pharmaceutics. 2024. PMID: 39204359 Free PMC article.
-
Nanoparticle Phototherapy in the Era of Cancer Immunotherapy.Trends Chem. 2020 Dec;2(12):1082-1095. doi: 10.1016/j.trechm.2020.09.008. Epub 2020 Oct 16. Trends Chem. 2020. PMID: 35178514 Free PMC article.
-
Recent Advances in Metal-Organic Framework (MOF)-Based Photocatalysts: Design Strategies and Applications in Heavy Metal Control.Molecules. 2023 Sep 18;28(18):6681. doi: 10.3390/molecules28186681. Molecules. 2023. PMID: 37764456 Free PMC article. Review.
References
-
- Ackroyd R, Kelty C, Brown N, Reed M. Photochemistry and Photobiology. 2001;74:656–669. - PubMed
-
- Pushpan S, Venkatraman S, Anand V, Sankar J, Parmeswaran D, Ganesan S, Chandrashekar T. Current Medicinal Chemistry-Anti-Cancer Agents. 2002;2:187–207. - PubMed
-
- Roelandts R. Journal of the American Academy of Dermatology. 2002;46:926–930. - PubMed
-
- Møller KI, Kongshoj B, Philipsen PA, Thomsen VO, Wulf HC. Photodermatology, Photoimmunology & Photomedicine. 2005;21:118–124. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
