Potential Role of Mic60/Mitofilin in Parkinson's Disease
- PMID: 30740041
- PMCID: PMC6357844
- DOI: 10.3389/fnins.2018.00898
Potential Role of Mic60/Mitofilin in Parkinson's Disease
Abstract
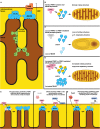
There are currently no treatments that hinder or halt the inexorable progression of Parkinson's disease (PD). While the etiology of PD remains elusive, evidence suggests that early dysfunction of mitochondrial respiration and homeostasis play a major role in PD pathogenesis. The mitochondrial structural protein Mic60, also known as mitofilin, is critical for maintaining mitochondrial architecture and function. Loss of Mic60 is associated with detrimental effects on mitochondrial homeostasis. Growing evidence now implicates Mic60 in the pathogenesis of PD. In this review, we discuss the data supporting a role of Mic60 and mitochondrial dysfunction in PD. We will also consider the potential of Mic60 as a therapeutic target for treating neurological disorders.
Keywords: Mic60/mitofilin; Parkinson’s disease; mitochondria; mitochondrial dynamics; neurodegeneration.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

