Schwann Cell Precursors Generate the Majority of Chromaffin Cells in Zuckerkandl Organ and Some Sympathetic Neurons in Paraganglia
- PMID: 30740044
- PMCID: PMC6355685
- DOI: 10.3389/fnmol.2019.00006
Schwann Cell Precursors Generate the Majority of Chromaffin Cells in Zuckerkandl Organ and Some Sympathetic Neurons in Paraganglia
Abstract
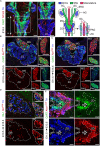
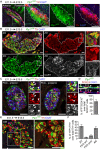
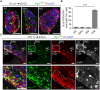
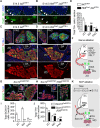
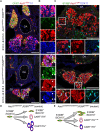
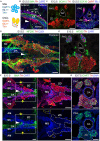
In humans, neurosecretory chromaffin cells control a number of important bodily functions, including those related to stress response. Chromaffin cells appear as a distinct cell type at the beginning of midgestation and are the main cellular source of adrenalin and noradrenalin released into the blood stream. In mammals, two different chromaffin organs emerge at a close distance to each other, the adrenal gland and Zuckerkandl organ (ZO). These two structures are found in close proximity to the kidneys and dorsal aorta, in a region where paraganglioma, pheochromocytoma and neuroblastoma originate in the majority of clinical cases. Recent studies showed that the chromaffin cells comprising the adrenal medulla are largely derived from nerve-associated multipotent Schwann cell precursors (SCPs) arriving at the adrenal anlage with the preganglionic nerve fibers, whereas the migratory neural crest cells provide only minor contribution. However, the embryonic origin of the ZO, which differs from the adrenal medulla in a number of aspects, has not been studied in detail. The ZO is composed of chromaffin cells in direct contact with the dorsal aorta and the intraperitoneal cavity and disappears through an autophagy-mediated mechanism after birth. In contrast, the adrenal medulla remains throughout the entire life and furthermore, is covered by the adrenal cortex. Using a combination of lineage tracing strategies with nerve- and cell type-specific ablations, we reveal that the ZO is largely SCP-derived and forms in synchrony with progressively increasing innervation. Moreover, the ZO develops hand-in-hand with the adjacent sympathetic ganglia that coalesce around the dorsal aorta. Finally, we were able to provide evidence for a SCP-contribution to a small but significant proportion of sympathetic neurons of the posterior paraganglia. Thus, this cellular source complements the neural crest, which acts as a main source of sympathetic neurons. Our discovery of a nerve-dependent origin of chromaffin cells and some sympathoblasts may help to understand the origin of pheochromocytoma, paraganglioma and neuroblastoma, all of which are currently thought to be derived from the neural crest or committed sympathoadrenal precursors.
Keywords: Schwann cell precursors; Zuckerkandl organ; catecholamines; extra-adrenal chromaffin cells; para-aortic sympathetic ganglia; posterior trunk sympathetic ganglia.
Figures
References
-
- Altergott R., Barbato A., Lawrence A., Paloyan E., Freeark R. J., Prinz R. A. (1985). Spectrum of catecholamine-secreting tumors of the organ of Zuckerkandl. Surgery 98 1121–1126. - PubMed
-
- Böck P. (1982). “The paraganglia,” in Handbuch der mikroskopischen Anatomie des Menschen, Vol. VI, eds Oksche A., Vollrath L. (Berlin: Springer; ). 10.1007/978-3-642-68208-7 - DOI
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

