Glucocorticoid Induced Leucine Zipper in Lipopolysaccharide Induced Neuroinflammation
- PMID: 30740047
- PMCID: PMC6355683
- DOI: 10.3389/fnagi.2018.00432
Glucocorticoid Induced Leucine Zipper in Lipopolysaccharide Induced Neuroinflammation
Abstract
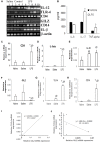
Glucocorticoids (GCs) are steroid hormones secreted as the end-product of the neuroendocrine stress cascade. Both absence and elevated GC mediate neurotoxic responses, suggesting that a narrow window ranging from physiological to slightly high GC mediate protective responses. The beneficial effects of GC are attributed to the transactivation of regulatory proteins and inhibition mediated by glucocorticoid receptor (GR) interactions with other co-factors. The glucocorticoid induced leucine zipper (GILZ) is a gene strongly upregulated by GC and mediates many of the anti-inflammatory and anti-proliferative effects of GC. Although GILZ is constitutively expressed in many tissues including the brain, the expression has been shown to occur with varying dynamics suggesting that the local milieu modulates its expression with consequent effects on cellular responses. Here we investigated the expression profile of GILZ in lipopolysaccharide (LPS) mediated neuroinflammation model of Alzheimer's disease (AD). Our data suggest that the GILZ expression is downregulated in neuroinflammation correlating inversely with the pro-inflammatory cytokines and innate immune responses.
Keywords: Alzheimer’s disease; cytokines; glucocorticoid induced leucine zipper; neuroinflammation; toll like receptor-4.
Figures

References
-
- Bergann T., Fromm A., Borden S. A., Fromm M., Schulzke J. D. (2011). Glucocorticoid receptor is indispensable for physiological responses to aldosterone in epithelial Na+ channel induction via the mineralocorticoid receptor in a human colonic cell line. Eur. J. Cell Biol. 90, 432–439. 10.1016/j.ejcb.2011.01.001 - DOI - PubMed
-
- Berrebi D., Bruscoli S., Cohen N., Foussat A., Migliorati G., Bouchet-Delbos L., et al. (2003). Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood 101, 729–738. 10.1182/blood-2002-02-0538 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

