Relative Frequencies of Alloantigen-Specific Helper CD4 T Cells and B Cells Determine Mode of Antibody-Mediated Allograft Rejection
- PMID: 30740108
- PMCID: PMC6357941
- DOI: 10.3389/fimmu.2018.03039
Relative Frequencies of Alloantigen-Specific Helper CD4 T Cells and B Cells Determine Mode of Antibody-Mediated Allograft Rejection
Abstract
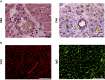
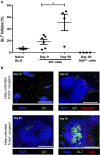
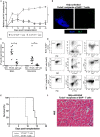
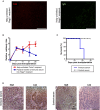
Humoral alloimmunity is now recognized as a major determinant of transplant outcome. MHC glycoprotein is considered a typical T-dependent antigen, but the nature of the T cell alloresponse that underpins alloantibody generation remains poorly understood. Here, we examine how the relative frequencies of alloantigen-specific B cells and helper CD4 T cells influence the humoral alloimmune response and how this relates to antibody-mediated rejection (AMR). An MHC-mismatched murine model of cardiac AMR was developed, in which T cell help for alloantibody responses in T cell deficient (Tcrbd-/-) C57BL/6 recipients against donor H-2Kd MHC class I alloantigen was provided by adoptively transferred "TCR75" CD4 T cells that recognize processed H-2Kd allopeptide via the indirect-pathway. Transfer of large numbers (5 × 105) of TCR75 CD4 T cells was associated with rapid development of robust class-switched anti-H-2Kd humoral alloimmunity and BALB/c heart grafts were rejected promptly (MST 9 days). Grafts were not rejected in T and B cell deficient Rag2-/- recipients that were reconstituted with TCR75 CD4 T cells or in control (non-reconstituted) Tcrbd-/- recipients, suggesting that the transferred TCR75 CD4 T cells were mediating graft rejection principally by providing help for effector alloantibody responses. In support, acutely rejecting BALB/c heart grafts exhibited hallmark features of acute AMR, with widespread complement C4d deposition, whereas cellular rejection was not evident. In addition, passive transfer of immune serum from rejecting mice to Rag2-/- recipients resulted in eventual BALB/c heart allograft rejection (MST 20 days). Despite being long-lived, the alloantibody responses observed at rejection of the BALB/c heart grafts were predominantly generated by extrafollicular foci: splenic germinal center (GC) activity had not yet developed; IgG secreting cells were confined to the splenic red pulp and bridging channels; and, most convincingly, rapid graft rejection still occurred when recipients were reconstituted with similar numbers of Sh2d1a-/- TCR75 CD4 T cells that are genetically incapable of providing T follicular helper cell function for generating GC alloimmunity. Similarly, alloantibody responses generated in Tcrbd-/- recipients reconstituted with smaller number of wild-type TCR75 CD4 T cells (103), although long-lasting, did not have a discernible extrafollicular component, and grafts were rejected much more slowly (MST 50 days). By modeling antibody responses to Hen Egg Lysozyme protein, we confirm that a high ratio of antigen-specific helper T cells to B cells favors development of the extrafollicular response, whereas GC activity is favored by a relatively high ratio of B cells. In summary, a relative abundance of helper CD4 T cells favors development of strong extrafollicular alloantibody responses that mediate acute humoral rejection, without requirement for GC activity. This work is composed of two parts, of which this is Part I. Please read also Part II: Chhabra et al., 2019.
Keywords: allograft; extrafollicular B cell response; germinal center (GC); humoral alloimmunity; transplantation; vasculopathy.
Figures


References
-
- Tan CD, Sokos GG, Pidwell DJ, Smedira NG, Gonzalez-Stawinski GV, Taylor DO, et al. Correlation of donor-specific antibodies, complement and its regulators with graft dysfunction in cardiac antibody-mediated rejection. Am J Transplant. (2009) 9:2075–84. 10.1111/j.1600-6143.2009.02748.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

