Therapeutic Cancer Vaccines-T Cell Responses and Epigenetic Modulation
- PMID: 30740111
- PMCID: PMC6357987
- DOI: 10.3389/fimmu.2018.03109
Therapeutic Cancer Vaccines-T Cell Responses and Epigenetic Modulation
Abstract
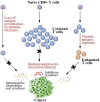
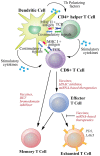
There is great interest in developing efficient therapeutic cancer vaccines, as this type of therapy allows targeted killing of tumor cells as well as long-lasting immune protection. High levels of tumor-infiltrating CD8+ T cells are associated with better prognosis in many cancers, and it is expected that new generation vaccines will induce effective production of these cells. Epigenetic mechanisms can promote changes in host immune responses, as well as mediate immune evasion by cancer cells. Here, we focus on epigenetic modifications involved in both vaccine-adjuvant-generated T cell immunity and cancer immune escape mechanisms. We propose that vaccine-adjuvant systems may be utilized to induce beneficial epigenetic modifications and discuss how epigenetic interventions could improve vaccine-based therapies. Additionally, we speculate on how, given the unique nature of individual epigenetic landscapes, epigenetic mapping of cancer progression and specific subsequent immune responses, could be harnessed to tailor therapeutic vaccines to each patient.
Keywords: DNA methylation; T cells; biomarkers; cancer vaccine-adjuvants; epigenetics; histone modifications; long non-coding RNAs; microRNAs.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

