A mechanistic roadmap for the clinical application of cardiac cell therapies
- PMID: 30740264
- PMCID: PMC6366940
- DOI: 10.1038/s41551-018-0216-z
A mechanistic roadmap for the clinical application of cardiac cell therapies
Abstract
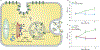
The development of cells for regenerative therapy has encountered many pitfalls on its path to clinical translation. In cardiology, clinical studies of heart-targeted cell therapies began two decades ago, yet progress towards reaching an approved product has been slow. In this Perspective, I provide an overview of recent cardiac cell therapies, with a focus on the hurdles limiting the translation of cell products from research laboratories to clinical practice. By focusing on heart failure as a target indication, I argue that strategies for overcoming limitations in clinical translation require an increasing emphasis on mechanism-supported efficacy, rather than on phenomenological observations. As research progresses from cells to paracrine mechanisms to defined factors, identifying those defined factors that are involved in achieving superior therapeutic efficacy will better inform the use of cells as therapeutic candidates. The next generation of cell-free biologics may provide the benefits of cell therapy without the intrinsic limitations of whole-cell products.
Conflict of interest statement
Competing interests E.M. holds founder’s equity in, and serves as unpaid scientific advisor to, Capricor Inc.
Figures




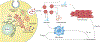
References
-
- Menasche P et al. Autologous skeletal myoblast transplantation for cardiac insufficiency. First Clin. Case. Arch. Mal. Coeur. Vaiss 94, 180–182 (2001). - PubMed
-
- Voronov RA Experimental study of the regenerative potentialities of the cardiac and somatic musculatures. Arkh. Anat. Gistol. Embriol 69, 35–40 (1975). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

