Manganese-Zinc Ferrites: Safe and Efficient Nanolabels for Cell Imaging and Tracking In Vivo
- PMID: 30740290
- PMCID: PMC6356160
- DOI: 10.1002/open.201800261
Manganese-Zinc Ferrites: Safe and Efficient Nanolabels for Cell Imaging and Tracking In Vivo
Abstract
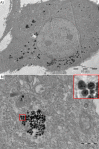
Manganese-zinc ferrite nanoparticles were synthesized by using a hydrothermal treatment, coated with silica, and then tested as efficient cellular labels for cell tracking, using magnetic resonance imaging (MRI) in vivo. A toxicity study was performed on rat mesenchymal stem cells and C6 glioblastoma cells. Adverse effects on viability and cell proliferation were observed at the highest concentration (0.55 mM) only; cell viability was not compromised at lower concentrations. Nanoparticle internalization was confirmed by transmission electron microscopy. The particles were found in membranous vesicles inside the cytoplasm. Although the metal content (0.42 pg Fe/cell) was lower compared to commercially available iron oxide nanoparticles, labeled cells reached a comparable relaxation rate R 2, owing to higher nanoparticle relaxivity. Cells from transgenic luciferase-positive rats were used for in vivo experiments. Labeled cells were transplanted into the muscles of non-bioluminescent rats and visualized by MRI. The cells produced a distinct hypointense signal in T2- or T2*-weighted MR images in vivo. Cell viability in vivo was verified by bioluminescence.
Keywords: cell labeling; cell transplantation; doping; magnetic resonance imaging; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials

