Advances in Computational Human Phantoms and Their Applications in Biomedical Engineering - A Topical Review
- PMID: 30740582
- PMCID: PMC6362464
- DOI: 10.1109/TRPMS.2018.2883437
Advances in Computational Human Phantoms and Their Applications in Biomedical Engineering - A Topical Review
Abstract
Over the past decades, significant improvements have been made in the field of computational human phantoms (CHPs) and their applications in biomedical engineering. Their sophistication has dramatically increased. The very first CHPs were composed of simple geometric volumes, e.g., cylinders and spheres, while current CHPs have a high resolution, cover a substantial range of the patient population, have high anatomical accuracy, are poseable, morphable, and are augmented with various details to perform functionalized computations. Advances in imaging techniques and semi-automated segmentation tools allow fast and personalized development of CHPs. These advances open the door to quickly develop personalized CHPs, inherently including the disease of the patient. Because many of these CHPs are increasingly providing data for regulatory submissions of various medical devices, the validity, anatomical accuracy, and availability to cover the entire patient population is of utmost importance. The article is organized into two main sections: the first section reviews the different modeling techniques used to create CHPs, whereas the second section discusses various applications of CHPs in biomedical engineering. Each topic gives an overview, a brief history, recent developments, and an outlook into the future.
Keywords: Human anatomy; computational modeling; phantoms.
Figures
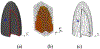
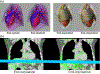
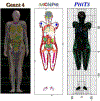
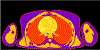

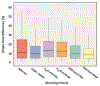
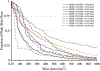
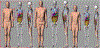


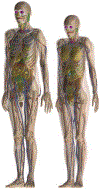

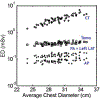


References
-
- Agostinelli S et al., “Geant4—a simulation toolkit,” Nucl. Instr. Meth. Phys. Res. Sec. A, vol. 506, no. 3, pp. 250–303, July 2003.
-
- Leyton M, A generative theory of shape, vol. 2145, Berlin: Springer-Verlag, November 2001.
-
- Stroud I, “Boundary representation modeling techniques”, London: Springer-Verlag, July 2006.
-
- Cristy M and Eckerman KF, “Specific absorbed fractions of energy at various ages from internal photon sources, Part I: Methods,” Oak Ridge National Laboratory, Oak Ridge, TN, USA, 1987.
-
- Xu XG and Eckerman KF, “Handbook of anatomical models for radiation dosimetry”, Taylor & Francis, 2009.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
