Bioengineering the microanatomy of human skin
- PMID: 30740672
- PMCID: PMC6422806
- DOI: 10.1111/joa.12942
Bioengineering the microanatomy of human skin
Abstract
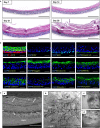
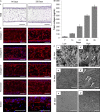
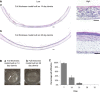
Recreating the structure of human tissues in the laboratory is valuable for fundamental research, testing interventions, and reducing the use of animals. Critical to the use of such technology is the ability to produce tissue models that accurately reproduce the microanatomy of the native tissue. Current artificial cell-based skin systems lack thorough characterisation, are not representative of human skin, and can show variation. In this study, we have developed a novel full thickness model of human skin comprised of epidermal and dermal compartments. Using an inert porous scaffold, we created a dermal construct using human fibroblasts that secrete their own extracellular matrix proteins, which avoids the use of animal-derived materials. The dermal construct acts as a foundation upon which epidermal keratinocytes were seeded and differentiated into a stratified keratinised epithelium. In-depth morphological analyses of the model demonstrated very close similarities with native human skin. Extensive immunostaining and electron microscopy analysis revealed ultrastructural details such as keratohyalin granules and lamellar bodies within the stratum granulosum, specialised junctional complexes, and the presence of a basal lamina. These features reflect the functional characteristics and barrier properties of the skin equivalent. Robustness and reproducibility of in vitro models are important attributes in experimental practice, and we demonstrate the consistency of the skin construct between different users. In summary, a new model of full thickness human skin has been developed that possesses microanatomical features reminiscent of native tissue. This skin model platform will be of significant interest to scientists researching the structure and function of human skin.
Keywords: barrier function; dermis; epidermis; human; methodology; reproducible; skin equivalent; tissue engineering.
© 2019 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Figures





References
-
- Ackermann K, Borgia SL, Korting HC, et al. (2010) The Phenion full‐thickness skin model for percutaneous absorption testing. Skin Pharmacol Physiol 23, 105–112. - PubMed
-
- Andreadis ST, Hamoen KE, Yarmush ML, et al. (2001) Keratinocyte growth factor induces hyperproliferation and delays differentiation in a skin equivalent model system. FASEB J 15, 898–906. - PubMed
-
- Auxenfans C, Fradette J, Lequeux C, et al. (2009) Evolution of three dimensional skin equivalent models reconstructed in vitro by tissue engineering. Eur J Dermatol 19, 107–113. - PubMed
-
- Bertolero F, Kaighn ME, Gonda MA, et al. (1984) Mouse epidermal keratinocytes. Clonal proliferation and response to hormones and growth factors in serum‐free medium. Exp Cell Res 155, 64–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

