Effect of adenosine on short-term synaptic plasticity in mouse piriform cortex in vitro: adenosine acts as a high-pass filter
- PMID: 30740934
- PMCID: PMC6369103
- DOI: 10.14814/phy2.13992
Effect of adenosine on short-term synaptic plasticity in mouse piriform cortex in vitro: adenosine acts as a high-pass filter
Abstract
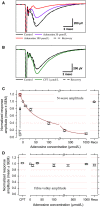
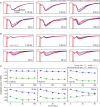
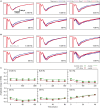
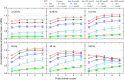
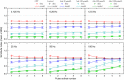
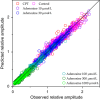
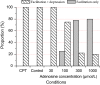
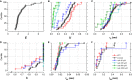
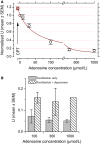
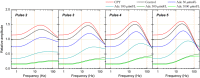
We examined the effect of adenosine and of adenosine A1 receptor blockage on short-term synaptic plasticity in slices of adult mouse anterior piriform cortex maintained in vitro in an in vivo-like ACSF. Extracellular recording of postsynaptic responses was performed in layer 1a while repeated electrical stimulation (5-pulse-trains, frequency between 3.125 and 100 Hz) was applied to the lateral olfactory tract. Our stimulation protocol was aimed at covering the frequency range of oscillatory activities observed in the olfactory bulb in vivo. In control condition, postsynaptic response amplitude showed a large enhancement for stimulation frequencies in the beta and gamma frequency range. A phenomenological model of short-term synaptic plasticity fitted to the data suggests that this frequency-dependent enhancement can be explained by the interplay between a short-term facilitation mechanism and two short-term depression mechanisms, with fast and slow recovery time constants. In the presence of adenosine, response amplitude evoked by low-frequency stimulation decreased in a dose-dependent manner (IC50 = 70 μmol/L). Yet short-term plasticity became more dominated by facilitation and less influenced by depression. Both changes compensated for the initial decrease in response amplitude in a way that depended on stimulation frequency: compensation was strongest at high frequency, up to restoring response amplitudes to values similar to those measured in control condition. The model suggested that the main effects of adenosine were to decrease neurotransmitter release probability and to attenuate short-term depression mechanisms. Overall, these results suggest that adenosine does not merely inhibit neuronal activity but acts in a more subtle, frequency-dependent manner.
Keywords: A1 receptor; oscillation; piriform cortex; presynaptic inhibition; short-term plasticity.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures

References
-
- Adrian, E. D. 1950. The electrical activity of the mammalian olfactory bulb. Electroencephalogr. Clin. Neurophysiol. 2:377–388. - PubMed
-
- Ballarín, M. , Fredholm B. B., Ambrosio S., and Mahy N.. 1991. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol. Scand. 142:97–103. - PubMed
-
- Berger, H. 1929. Über das elektrenkephalogramm des menschen. Arch. Psychiatr. Nervenkr. 87:527–570.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

