Bioinspired Fabrication of DNA-Inorganic Hybrid Composites Using Synthetic DNA
- PMID: 30741535
- PMCID: PMC6439439
- DOI: 10.1021/acsnano.8b06492
Bioinspired Fabrication of DNA-Inorganic Hybrid Composites Using Synthetic DNA
Abstract
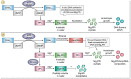
Nucleic acid nanostructures have attracted significant interest as potential therapeutic and diagnostic platforms due to their intrinsic biocompatibility and biodegradability, structural and functional diversity, and compatibility with various chemistries for modification and stabilization. Among the fabrication approaches for such structures, the rolling circle techniques have emerged as particularly promising, producing morphologically round, flower-shaped nucleic acid particles: typically hybrid composites of long nucleic acid strands and inorganic magnesium pyrophosphate (Mg2PPi). These constructs are known to form via anisotropic nucleic acid-driven crystallization in a sequence-independent manner, rendering monodisperse and densely packed RNA or DNA-inorganic composites. However, it still remains to fully explore how flexible polymer-like RNA or DNA strands (acting as biomolecular additives) mediate the crystallization process of Mg2PPi and affect the structure and properties of the product crystals. To address this, we closely examined nanoscale details to mesoscopic features of Mg2PPi/DNA hybrid composites fabricated by two approaches, namely rolling circle amplification (RCA)-based in situ synthesis and long synthetic DNA-mediated crystallization. Similar to the DNA constructs fabricated by RCA, the rapid crystallization of Mg2PPi was retarded on a short-range order when we precipitated the crystals in the presence of presynthesized long DNA, which resulted in effective incorporation of biomolecular additives such as DNA and enzymes. These findings further provide a more feasible way to encapsulate bioactive enzymes within DNA constructs compared to in situ RCA-mediated synthesis, i.e., by not only protecting them from possible denaturation under the reaction conditions but also preventing nonselective association of proteins arising from the RCA reaction mixtures.
Keywords: DNA inclusion; DNA-inorganic hybrid composites; coprecipitation; crystallization; rolling circle techniques.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Heuer A. H.; Fink D. J.; Laraia V. J.; Arias J. L.; Calvert P. D.; Kendall K.; Messing G. L.; Blackwell J.; Rieke P. C.; Thompson D. H.; Wheeler A. P.; Veis A.; Caplan A. I. Innovative Materials Processing Strategies: A Biomimetic Approach. Science 1992, 255, 1098–1105. 10.1126/science.1546311. - DOI - PubMed
-
- Song R.-Q.; Colfen H. Additive Controlled Crystallization. CrystEngComm 2011, 13, 1249–1276. 10.1039/c0ce00419g. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

