MG 53 Protein Protects Aortic Valve Interstitial Cells From Membrane Injury and Fibrocalcific Remodeling
- PMID: 30741589
- PMCID: PMC6405656
- DOI: 10.1161/JAHA.118.009960
MG 53 Protein Protects Aortic Valve Interstitial Cells From Membrane Injury and Fibrocalcific Remodeling
Erratum in
-
Correction to: MG53 Protein Protects Aortic Valve Interstitial Cells From Membrane Injury and Fibrocalcific Remodeling.J Am Heart Assoc. 2020 Jun 16;9(12):e014526. doi: 10.1161/JAHA.119.014526. Epub 2020 Jun 16. J Am Heart Assoc. 2020. PMID: 32539606 Free PMC article. No abstract available.
Abstract
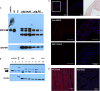
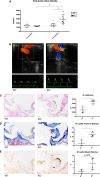
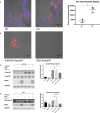
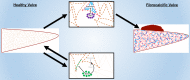
Background The aortic valve of the heart experiences constant mechanical stress under physiological conditions. Maladaptive valve injury responses contribute to the development of valvular heart disease. Here, we test the hypothesis that MG 53 (mitsugumin 53), an essential cell membrane repair protein, can protect valvular cells from injury and fibrocalcific remodeling processes associated with valvular heart disease. Methods and Results We found that MG 53 is expressed in pig and human patient aortic valves and observed aortic valve disease in aged Mg53-/- mice. Aortic valves of Mg53-/- mice showed compromised cell membrane integrity. In vitro studies demonstrated that recombinant human MG 53 protein protects primary valve interstitial cells from mechanical injury and that, in addition to mediating membrane repair, recombinant human MG 53 can enter valve interstitial cells and suppress transforming growth factor-β-dependent activation of fibrocalcific signaling. Conclusions Together, our data characterize valve interstitial cell membrane repair as a novel mechanism of protection against valvular remodeling and assess potential in vivo roles of MG 53 in preventing valvular heart disease.
Keywords: cell membrane repair; fibrosis; heart valve; transforming growth factor‐β; valvular heart disease.
Figures

References
-
- Lester WM, Gotlieb AI. In vitro repair of the wounded porcine mitral valve. Circ Res. 1988;62:833–845. - PubMed
-
- Lester WM, Damji AA, Tanaka M, Gedeon I. Bovine mitral valve organ culture: role of interstitial cells in repair of valvular injury. J Mol Cell Cardiol. 1992;24:43–53. - PubMed
-
- Lester WM, Damji AA, Gedeon I, Tanaka M. Interstitial cells from the atrial and ventricular sides of the bovine mitral valve respond differently to denuding endocardial injury. In Vitro Cell Dev Biol. 1993;29A:41–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

