Effect of atorvastatin on iron metabolism regulation in patients with chronic kidney disease - a randomized double blind crossover study
- PMID: 30741616
- PMCID: PMC6319462
- DOI: 10.1080/0886022X.2018.1535983
Effect of atorvastatin on iron metabolism regulation in patients with chronic kidney disease - a randomized double blind crossover study
Abstract
Introduction: To determine the effect of 6-month administration of atorvastatin on hepcidin and hemojuvelin levels, inflammatory parameters and iron metabolism in patients with chronic kidney disease (CKD) stages 3 and 4.
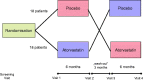
Methods: Thirty six statin- and erythropoiesis-stimulating agent-naive patients with CKD stages 3 and 4 and LDL cholesterol ≥100 mg/dl received atorvastatin or placebo for two 6-month periods in a double blind, randomized crossover study. Hepcidin, hemojuvelin, hsCRP, IL-6, hemoglobin, red blood cell distribution width, iron, total iron binding capacity (TIBC), and unsaturated iron binding capacity (UIBC) were measured before and after each treatment period.
Results: Hepcidin decreased (from 102 [307] to 63 [170] pg/ml (p > .001)) in the course of statin therapy but remained unchanged after placebo administration (173 [256] to 153 [204] pg/ml, respectively). Hemojuvelin did not change after either part of the study. Both IL-6 and hsCRP decreased following statin therapy (from 8.7 [12.0] to 8.1 [13.9] pg/ml; p = .04 and from 4.7 [4.0] to 4.0 [3.6] mg/l; p = .4, respectively), but did not change after placebo administration. Blood hemoglobin increased slightly but significantly after 6-month statin therapy (from 11.6 ± 1.6 to 11.9 ± 1.5 g/dl, p = .002), and was unchanged after placebo treatment. TIBC and UIBC increased significantly after 6-month statin therapy, and serum iron also tended to increase. The change of eGFR during the study did not differ between the two treatment periods.
Conclusions: Statin may have a small but potentially beneficial effect on serum hepcidin, which may lead to improvement of anemia control in CKD patients.
Keywords: Hepcidin; chronic kidney disease; hemojuvelin; inflammation; iron metabolism.
Figures
References
-
- Besarab A, Levin A. Defining a renal anemia management period. Am J Kidney Dis. 2000;36:S13–S23. - PubMed
-
- Deicher R, Horl WH. New insights into the regulation of iron homeostasis. Eur J Clin Invest. 2006;36:301–309. - PubMed
-
- Swinkels DW, Wetzels JFM. Hepcidin: a new tool in the management of anaemia in patients with chronic kidney disease? Nephrol Dial Transplant. 2008;23:2450–2453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous