Neurotrophins and their involvement in digestive cancers
- PMID: 30741921
- PMCID: PMC6370832
- DOI: 10.1038/s41419-019-1385-8
Neurotrophins and their involvement in digestive cancers
Abstract
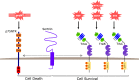
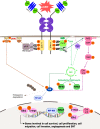
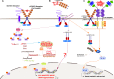
Cancers of the digestive system, including esophageal, gastric, pancreatic, hepatic, and colorectal cancers, have a high incidence and mortality worldwide. Efficient therapies have improved patient care; however, many challenges remain including late diagnosis, disease recurrence, and resistance to therapies. Mechanisms responsible for these aforementioned challenges are numerous. This review focuses on neurotrophins, including NGF, BDNF, and NT3, and their specific tyrosine kinase receptors called tropomyosin receptor kinase (Trk A, B, C, respectively), associated with sortilin and the p75 neurotrophin receptor (p75NTR), and their implication in digestive cancers. Globally, p75NTR is a frequently downregulated tumor suppressor. On the contrary, Trk and their ligands are considered oncogenic factors. New therapies which target NT and/or their receptors, or use them as diagnosis biomarkers could help us to combat digestive cancers.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

