Molecular layer interneurons shape the spike activity of cerebellar Purkinje cells
- PMID: 30742002
- PMCID: PMC6370775
- DOI: 10.1038/s41598-018-38264-1
Molecular layer interneurons shape the spike activity of cerebellar Purkinje cells
Abstract
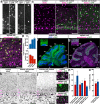
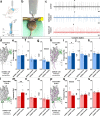
Purkinje cells receive synaptic input from several classes of interneurons. Here, we address the roles of inhibitory molecular layer interneurons in establishing Purkinje cell function in vivo. Using conditional genetics approaches in mice, we compare how the lack of stellate cell versus basket cell GABAergic neurotransmission sculpts the firing properties of Purkinje cells. We take advantage of an inducible Ascl1CreER allele to spatially and temporally target the deletion of the vesicular GABA transporter, Vgat, in developing neurons. Selective depletion of basket cell GABAergic neurotransmission increases the frequency of Purkinje cell simple spike firing and decreases the frequency of complex spike firing in adult behaving mice. In contrast, lack of stellate cell communication increases the regularity of Purkinje cell simple spike firing while increasing the frequency of complex spike firing. Our data uncover complementary roles for molecular layer interneurons in shaping the rate and pattern of Purkinje cell activity in vivo.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Eccles JC, Llinás R, Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp. brain Res. 1966;1:17–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

