Japanese encephalitis virus neuropenetrance is driven by mast cell chymase
- PMID: 30742008
- PMCID: PMC6370868
- DOI: 10.1038/s41467-019-08641-z
Japanese encephalitis virus neuropenetrance is driven by mast cell chymase
Abstract
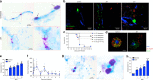
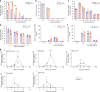
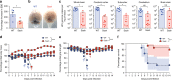
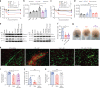
Japanese encephalitis virus (JEV) is a leading cause of viral encephalitis. However, the mechanisms of JEV penetration of the blood-brain-barrier (BBB) remain poorly understood. Mast cells (MCs) are granulated innate immune sentinels located perivascularly, including at the BBB. Here we show that JEV activates MCs, leading to the release of granule-associated proteases in vivo. MC-deficient mice display reduced BBB permeability during JEV infection compared to congenic wild-type (WT) mice, indicating that enhanced vascular leakage in the brain during JEV infection is MC-dependent. Moreover, MCs promoted increased JEV infection in the central nervous system (CNS), enhanced neurological deficits, and reduced survival in vivo. Mechanistically, chymase, a MC-specific protease, enhances JEV-induced breakdown of the BBB and cleavage of tight-junction proteins. Chymase inhibition reversed BBB leakage, reduced brain infection and neurological deficits during JEV infection, and prolonged survival, suggesting chymase is a novel therapeutic target to prevent JEV encephalitis.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Viral Infection of the Central Nervous System and Neuroinflammation Precede Blood-Brain Barrier Disruption during Japanese Encephalitis Virus Infection.J Virol. 2015 May;89(10):5602-14. doi: 10.1128/JVI.00143-15. Epub 2015 Mar 11. J Virol. 2015. PMID: 25762733 Free PMC article.
-
Ablation of CD11c(hi) dendritic cells exacerbates Japanese encephalitis by regulating blood-brain barrier permeability and altering tight junction/adhesion molecules.Comp Immunol Microbiol Infect Dis. 2016 Oct;48:22-32. doi: 10.1016/j.cimid.2016.07.007. Epub 2016 Jul 26. Comp Immunol Microbiol Infect Dis. 2016. PMID: 27638116
-
CLEC5A regulates Japanese encephalitis virus-induced neuroinflammation and lethality.PLoS Pathog. 2012;8(4):e1002655. doi: 10.1371/journal.ppat.1002655. Epub 2012 Apr 19. PLoS Pathog. 2012. PMID: 22536153 Free PMC article.
-
Molecular Mechanism and Role of Japanese Encephalitis Virus Infection in Central Nervous System-Mediated Diseases.Viruses. 2022 Nov 30;14(12):2686. doi: 10.3390/v14122686. Viruses. 2022. PMID: 36560690 Free PMC article. Review.
-
Japanese encephalitis and Japanese encephalitis virus in mainland China.Rev Med Virol. 2012 Sep;22(5):301-22. doi: 10.1002/rmv.1710. Epub 2012 Mar 8. Rev Med Virol. 2012. PMID: 22407526 Review.
Cited by
-
Comparative analysis of neuroinvasion by Japanese encephalitis virulent and vaccine viral strains in an in vitro model of human blood-brain barrier.PLoS One. 2021 Jun 4;16(6):e0252595. doi: 10.1371/journal.pone.0252595. eCollection 2021. PLoS One. 2021. PMID: 34086776 Free PMC article.
-
GAS6 as a potential target to alleviate neuroinflammation during Japanese encephalitis in mouse models.J Neuroinflammation. 2024 Sep 19;21(1):231. doi: 10.1186/s12974-024-03225-1. J Neuroinflammation. 2024. PMID: 39300526 Free PMC article.
-
Review of Emerging Japanese Encephalitis Virus: New Aspects and Concepts about Entry into the Brain and Inter-Cellular Spreading.Pathogens. 2019 Jul 26;8(3):111. doi: 10.3390/pathogens8030111. Pathogens. 2019. PMID: 31357540 Free PMC article. Review.
-
Flavivirus NS1 Triggers Tissue-Specific Disassembly of Intercellular Junctions Leading to Barrier Dysfunction and Vascular Leak in a GSK-3β-Dependent Manner.Pathogens. 2022 May 24;11(6):615. doi: 10.3390/pathogens11060615. Pathogens. 2022. PMID: 35745469 Free PMC article.
-
Responses of Mast Cells to Pathogens: Beneficial and Detrimental Roles.Front Immunol. 2021 Jun 15;12:685865. doi: 10.3389/fimmu.2021.685865. eCollection 2021. Front Immunol. 2021. PMID: 34211473 Free PMC article. Review.
References
-
- Solomon T, Vaughn DW. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr. Top. Microbiol. Immunol. 2002;267:171–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

