MAPT mutations, tauopathy, and mechanisms of neurodegeneration
- PMID: 30742061
- PMCID: PMC7289372
- DOI: 10.1038/s41374-019-0197-x
MAPT mutations, tauopathy, and mechanisms of neurodegeneration
Abstract
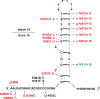
In multiple neurodegenerative diseases, including Alzheimer's disease (AD), a prominent pathological feature is the aberrant aggregation and inclusion formation of the microtubule-associated protein tau. Because of the pathological association, these disorders are often referred to as tauopathies. Mutations in the MAPT gene that encodes tau can cause frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17), providing the clearest evidence that tauopathy plays a causal role in neurodegeneration. However, large gaps in our knowledge remain regarding how various FTDP-17-linked tau mutations promote tau aggregation and neurodegeneration, and, more generally, how the tauopathy is linked to neurodegeneration. Herein, we review what is known about how FTDP-17-linked pathogenic MAPT mutations cause disease, with a major focus on the prion-like properties of wild-type and mutant tau proteins. The hypothesized mechanisms by which mutations in the MAPT gene promote tauopathy are quite varied and may not provide definitive insights into how tauopathy arises in the absence of mutation. Further, differences in the ability of tau and mutant tau proteins to support prion-like propagation in various model systems raise questions about the generalizability of this mechanism in various tauopathies. Notably, understanding the mechanisms of tauopathy induction and spread and tau-induced neurodegeneration has important implications for tau-targeting therapeutics.
Conflict of interest statement
Disclosure/Conflict of Interest
The authors declare that they have no conflicts of interest with the content of this article.
Figures
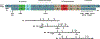
References
-
- Arendt T, Stieler JT, Holzer M. Tau and tauopathies. Brain Res Bull 2016;126:238–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

