Two-way communication between ex vivo tissues on a microfluidic chip: application to tumor-lymph node interaction
- PMID: 30742147
- PMCID: PMC6416076
- DOI: 10.1039/c8lc00957k
Two-way communication between ex vivo tissues on a microfluidic chip: application to tumor-lymph node interaction
Abstract
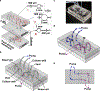
Experimentally accessible tools to replicate the complex biological events of in vivo organs offer the potential to reveal mechanisms of disease and potential routes to therapy. In particular, models of inter-organ communication are emerging as the next essential step towards creating a body-on-a-chip, and may be particularly useful for poorly understood processes such as tumor immunity. In this paper, we report the first multi-compartment microfluidic chip that continuously recirculates a small volume of media through two ex vivo tissue samples to support inter-organ cross-talk via secreted factors. To test on-chip communication, protein release and capture were quantified using well-defined artificial tissue samples and model proteins. Proteins released by one sample were transferred to the downstream reservoir and detectable in the downstream sample. Next, the chip was applied to model the communication between a tumor and a lymph node, to test whether on-chip dual-organ culture could recreate key features of tumor-induced immune suppression. Slices of murine lymph node were co-cultured with tumor or healthy tissue on-chip with recirculating media, then tested for their ability to respond to T cell stimulation. Interestingly, lymph node slices co-cultured with tumor slices appeared more immunosuppressed than those co-cultured with healthy tissue, suggesting that the chip may successfully model some features of tumor-immune interaction. In conclusion, this new microfluidic system provides on-chip co-culture of pairs of tissue slices under continuous recirculating flow, and has the potential to model complex inter-organ communication ex vivo with full experimental accessibility of the tissues and their media.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

