Combined hormonal contraceptives for heavy menstrual bleeding
- PMID: 30742315
- PMCID: PMC6369862
- DOI: 10.1002/14651858.CD000154.pub3
Combined hormonal contraceptives for heavy menstrual bleeding
Abstract
Background: Menorrhagia or heavy menstrual bleeding (HMB) is an excessive blood loss that impairs a woman's quality of life, either physical, emotional, social or material. It is benign and not associated with pregnancy or any other gynaecological or systemic disease. Medical treatments used to reduce excessive menstrual blood loss (MBL) include prostaglandin synthetase inhibitors, antifibrinolytics, oral contraceptive pills, and other hormones. The combined oral contraceptive pill (COCP) is claimed to have a variety of beneficial effects, inducing a regular shedding of a thinner endometrium and inhibiting ovulation, thus having the effect of both treating HMB and providing contraception. More recently, a contraceptive vaginal ring (CVR) has been trialled to investigate whether this treatment can provide similar benefits to COCP while lessening hormonal systemic exposure. This review is an update of a review which originally focused on COCP alone. The scope of the review has been widened to consider other types of delivery of combined hormonal contraceptives for reduction of MBL.
Objectives: To determine the efficacy of combined hormonal contraceptives (pills, vaginal ring or patch) compared with other medical therapies, placebo, or no therapy in the treatment of HMB. A secondary objective was to compare the COCP with the CVR.
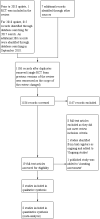
Search methods: We searched the Gynecology and Fertility Group trials register, MEDLINE, Embase, CENTRAL, CINAHL and PsycINFO (search dates: Oct 1996, May 2002, June 2004, April 2006, June 2009, July 2017 and September 2018) for all randomised controlled trials (RCTs) of COCP and CVR for the treatment of HMB. We also searched trial registers and the reference lists of retrieved studies for additional trials.
Selection criteria: We included randomised controlled trials (RCTs) of the use of COCP or CVR compared with no treatment, placebo, or other medical therapies for women with HMB and regular menstrual cycles.
Data collection and analysis: All assessments of trial quality and data extraction were performed unblinded by at least two review authors. Our primary review outcomes were treatment success, menstrual bleeding (assessed objectively, semi-objectively or subjectively), and participant satisfaction with treatment. Secondary outcomes were adverse events, quality of life, and haemoglobin level.
Main results: We identified eight RCTs involving 805 participants. Two trials comparing COCP with placebo were considered to be moderate quality and the remaining studies were low to very low quality, mainly because of serious risk of bias from lack of blinding and concerns over precision.COCP versus placeboCOCP, with a step-down oestrogen and step-up progestogen regimen, improved response to treatment (return to menstrual 'normality') (OR 22.12, 95% CI 4.40 to 111.12; 2 trials; 363 participants; I2 = 50%; moderate-quality evidence), and lowered MBL (OR 5.15, 95% CI 3.16 to 8.40; 2 trials; 339 participants; I2 = 0%; moderate-quality evidence) when compared to placebo. The results suggested that, if the chance of 'successful' treatment was 3% in women taking placebo, then COCP increased this chance from 12% to 77% in women with unacceptable HMB. Minor adverse events, in particular breast pain, were more common with COCP. No study in this comparison reported semi-objectively assessed MBL or participant satisfaction with treatment.COCP versus other medical treatmentsNon-steroidal anti-inflammatory drugs (NSAIDs)There was insufficient evidence to determine whether the COCP reduced MBL when compared to NSAIDs (mefenamic acid and naproxen). No study in this comparison reported semi-objectively assessed MBL, subjectively assessed MBL, participant satisfaction with treatment or adverse events.Levonorgestrel-releasing intrauterine system (LNG IUS)The LNG IUS was more effective than COCP in reducing MBL (OR 0.21, 95% CI 0.09 to 0.48; 2 trials; 151 participants; I2 = 0%; low-quality evidence) but it was not clear whether satisfaction with treatment or adverse effects varied according to which treatment was used. No study in this comparison reported semi-objectively assessed MBL or subjectively assessed MBL.Contraceptive vaginal ring (CVR) versus other medical treatmentsCOCP COCP was compared with CVR in two trials. There were discrepancies between some of the findings and there was no evidence of a benefit for one treatment compared to the other for response to treatment, MBL or participant satisfaction with treatment. There was a greater likelihood of nausea with COCP. No study in this comparison reported objectively assessed MBL or subjectively assessed MBL.ProgestogensCVR was compared to long course progestogens in one trial. It is possible that CVR increased the odds of satisfaction; but we are uncertain whether CVR improved MBL. The evidence was based on small numbers of participants and was very low quality, so definitive conclusions could not be reached. No study in this comparison reported objectively assessed MBL, subjectively assessed MBL, or adverse events.
Authors' conclusions: Moderate-quality evidence suggests that the combined oral contraceptive pill over six months reduces HMB in women with unacceptable HMB from 12% to 77% (compared to 3% in women taking placebo). When compared with other medical options for HMB, COCP was less effective than the LNG IUS. Limited evidence suggested that COCP and CVR had similar effects. There was insufficient evidence to determine comparative efficacy of combined hormonal contraceptives with NSAIDs, or long course progestogens.
Conflict of interest statement
AL, MWi, MWe, JB, CF and MB have no conflicts to declare.
Figures
Update of
-
Oral contraceptive pill for heavy menstrual bleeding.Cochrane Database Syst Rev. 2009 Oct 7;(4):CD000154. doi: 10.1002/14651858.CD000154.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2019 Feb 11;2:CD000154. doi: 10.1002/14651858.CD000154.pub3. PMID: 19821266 Updated.
References
References to studies included in this review
Agarwal 2016 {published data only}
-
- Agarwal N, Gupta M, Kriplani A, Bhatla N, Singh N. Comparison of combined hormonal vaginal ring with ultralow‐dose combined oral contraceptive pills in the management of heavy menstrual bleeding: a pilot study. Journal of Obstetrics & Gynecology 2016;36:71‐5. - PubMed
Dahiya 2016 {published data only}
-
- Dahiya P, Dalal M, Yadav A, Dahiya K, Jain S, Silan V. Efficacy of combined hormonal vaginal ring in comparison to combined hormonal pills in heavy menstrual bleeding. European Journal of Obstetrics & Gynecology and Reproductive Biology 2016;203:147‐51. [DOI: 10.1016/j.ejogrb.2016.05.009] - DOI - PubMed
Endrikat 2009 {published data only}
-
- Endrikat J, Shapiro H, Lukkari‐Lax E, Kunz M, Schmidt W, Fortier M. A Canadian, multicentre study comparing efficacy of a levonorgestrel‐releasing intrauterine system to an oral contraceptive in women with idiopathic menorrhagia. Journal of Obstetrics and Gynaecology Canada 2009;31(4):340‐7. - PubMed
Fraser 1991 {published data only}
-
- Fraser IS, McCarron G. Randomized trial of 2 hormonal and 2 prostaglandin‐inhibiting agents in women with a complaint of menorrhagia. Australian & New Zealand Journal of Obstetrics & Gynaecology 1991;31:66‐70. - PubMed
Fraser 2011 {published data only}
-
- Fraser IS, Romer T, Parke S, Zeun S, Mellinger U, Machlitt A, Jet al. Effective treatment of heavy and/or prolonged menstrual bleeding with an oral contraceptive containing estradiol valerate and dienogest: a randomized double‐blind Phase III trial. Human Reproduction 2011;26(10):2698‐708. [DOI: 10.1093/humrep/der224] - DOI - PubMed
-
- NCT00307801. Efficacy and safety study for the treatment of dysfunctional bleeding. clinicaltrials/ct2/show/results/NCT00307801 (first received 27 March 2006).
-
- Wasiak R, Filonenko A, Vanness DJ, Wittrup‐Jensen KU, Stull DE, Siak S, et al. Impact of estradiol‐valerate/dienogest on work productivity and activities of daily living in European and Australian women with heavy menstrual bleeding. International Journal of Women's Health 2012;4:271‐8. - PMC - PubMed
Hashim 2012 {published data only}
-
- Hashim HA, Alsherbini W, Bazeed M. Contraceptive vaginal ring treatment of heavy menstrual bleeding: a randomized controlled trial with norethisterone. Contraception 2012;85:246‐52. - PubMed
Jensen 2011 {published data only}
-
- NCT00293059. Efficacy and safety study for the treatment of dysfunctional uterine bleeding. clinicaltrials.gov.ct2/show/results/NCT00293059 (first received 15 February 2006).
-
- Wasiak R, Filonenko A, Vanness DJ, Law A, Jeddi M, Wittrup‐Jensen KU, et al. Impact of estradiol valerate/dienogest on work productivity and activities of daily living in women with heavy menstrual bleeding. Journal of Women's Health 2013;22(4):378‐84. - PubMed
Shabaan 2011 {published data only}
References to studies excluded from this review
Creatsas 1998 {published data only}
-
- Creatsas G, Cardamakis E, Deligeoroglou E, Hassan E, Tzingounis V. Tenoxicam versus lynestrenol‐ethinyl estradiol treatment of dysfunctional uterine bleeding cases during adolescence. Journal of Pediatric Adolescent Gynecology 1998;11:177‐80. - PubMed
Davis 2000 {published data only}
-
- Davis A, Godwin A, Lippman J, Olson W, Kafrissen M. Triphasic norgestimate‐ethinyl estradiol for treating dysfunctional uterine bleeding. Obstetrics and Gynecology 2000;96:913‐20. - PubMed
Jain 2016 {published data only}
-
- Jain S, Vaid NB, Narang Y, Suneja A, Guleria K. A randomised controlled trial comparing the efficacy and side‐effects of intravaginal ring (Nuvaring) with combined oral hormonal preparation in dysfunctional uterine bleeding. Journal of Clinical and Diagnostic Research 2016;10(3):QC21‐4. [DOI: 10.7860/JCDR/2016/16545.7516] - DOI - PMC - PubMed
Kriplani 2016 {published data only}
-
- Kriplani A, Srivastava A, Kulshrestha V, Kachawa G, Agarwal N, Bhatla N, et al. Efficacy of ormeloxifene versus oral contraceptive in the management of abnormal uterine bleeding due to uterine leiomyoma. Obstetrics and Gynaecology Research 2016;42(12):1744‐52. [DOI: 10.1111/jog.13105] - DOI - PubMed
Munro 2006 {published data only}
-
- Munro MG, Mainor N, Basu R, Brisinger M, Barreda L. Oral medroxyprogesterone acetate and combination oral contraceptives for acute uterine bleeding. Obstetrics & Gynecology 2006;108:924‐9. - PubMed
Sayed 2011 {published data only}
-
- Sayed GH, Zakherah MS, El‐Nashar SA, Shaaban MM. A randomized clinical trial of levonorgestrel‐releasi intrauterine system and a low‐dose combined oral contraceptive for fibroid‐related menorrhagia. International Journal of Gynecology and Obstetrics 2011;112:126‐30. [DOI: 10.1016/j.ijgo.2010.08.009] - DOI - PubMed
Srivaths 2015 {published data only}
-
- Srivaths LV, Dietrich JE, Yee DL, Sangi‐Haghpeykar H, Mahoney D Jr. Oral tranexamic acid versus combined oral contraceptives for adolescent heavy menstrual bleeding: a pilot study. Journal of Pediatric and Adolescent Gynecology 2015;28(4):254‐7. - PubMed
Weisberg 2015 {published data only}
-
- Weisberg E, Merki‐Field GS, McGeechan K, Fraser IS. Randomized comparison of bleeding patterns in women using a combined contraceptive vaginal ring or a low‐dose combined oral contraceptive on a menstrually signaled regimen. Contraception 2015;91:121‐6. - PubMed
References to studies awaiting assessment
Yu 2018 {published data only}
-
- Yu Q, Zhou Y, Suturina L, Jaisamram U, Lu D, Parke S. Efficacy and safety of estradiol valerate/dienogest for the management of heavy menstrual bleeding: a multicenter double‐blind randomized placebo‐controlled phase III trial. Journal of Women's Health 2018;27(10):1225‐32. - PubMed
-
- Yu Q, Zhou Y, Suturina L, Jaisamrarn U, Lu D, Parke S. Four‐phasic oral contraceptive for the treatment of heavy menstrual bleeding. clinicaltrials.gov/ct2/show/record/NCT01638923?term=NCT01638923&rank=1 (first received 12 July 2012).
References to ongoing studies
NCT02002260 {unpublished data only}
-
- NCT02002260. Stopping Heavy Periods Project (SHiPP). clinicaltrials.gov/ct2/show/record/NCT02002260 (first received 5 December 2013).
NCT02943655 {unpublished data only}
-
- NCT02943655. Treatment of heavy and/or prolonged menstrual bleeding without organic cause. clinicaltrials.gov/ct2/show/NCT02943655 (first received 25 October 2016).
Additional references
Bahamondes 2011
-
- Bahamondes L, Bahamondes MV. Use of combined oral contraceptives for the management of heavy menstrual bleeding. Expert Reviews Obstetrics and Gynecology 2011;6(5):485‐9.
Bjarnadottir 2002
-
- Bjarnadottir RI, Tuppurainen M, Kilick SP. Comparison of cycle control with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl estradiol. American Journal of Obstetrics & Gynecology 2002;186:389‐95. - PubMed
Bofill Rodriguez 2018
Bryant‐Smith 2018
Fraser 1985
-
- Fraser IS, McCarron G, Markham R, Rest T. Blood and total fluid content of menstrual discharge. Obstetrics & Gynaecology 1985;65:194‐8. - PubMed
Fraser 2009
-
- Fraser I, Langham S, Uhl‐Hochgraeber K. Health‐related quality of life and economic burden of abnormal uterine bleeding. Expert Review of Obstetrics & Gynecology 2009;4(2):179‐89. [DOI: 10.1586/17474108.4.2.179] - DOI
Fritz 2012
-
- Fritz MA, Speroff L (eds). Clinical Gynecologic Endocrinology and Infertility. Vol. 242, Philadelphia: Lippincott Williams & Wilkins, 2012.
GRADEpro GDT [Computer program]
-
- GRADE Working Group, McMaster University. GRADEproGDT. Version accessed prior to 4 June 2018. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hallberg 1966
-
- Hallberg L, Hogdahl AM, Nilson L, Rybo G. Menstrual blood loss ‐ a population study. Acta Obstetricia et Gynecologica Scandinavica 1966;45:320‐51. - PubMed
Haynes 1977
-
- Haynes P, Hodgson H, Anderson A, Turnbull A. Measurement of menstrual blood loss in patients complaining of menorrhagia. British Journal of Obstetrics & Gynaecology 1977;84:763‐8. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higham 1990
-
- Higham JM, O'Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart.. British Journal of Obstetrics and Gynaecology 1990;97(8):734‐9. [PUBMED: PMID: 2400752] - PubMed
Kaunitz 2009a
-
- Kaunitz AM, Burkman RT, Fisher AC, Laguardia KD. Cycle control with a 21‐day compared with a 24‐day oral contraceptive pill: a randomized controlled trial. Obstetrics & Gynecology 2009;14:1205‐12. - PubMed
Kaunitz 2009b
-
- Kaunitz AM, Portman DJ, Hait H, Reape KZ. Adding low‐dose estrogen to the hormone‐free interval: impact on bleeding patterns in users of a 91‐day extended regimen of oral contraceptives. Contraception 2009;79:350‐5. - PubMed
Lethaby 2008
Lethaby 2013
Lethaby 2015
Liu 2007
-
- Liu Z, Doan Q, Blumenthal P, Dubois R. A systematic review evaluating health‐related quality of life, work impairment, and health‐care costs and utilization in abnormal uterine bleeding. Value Health 2007;10(3):183‐94. [10.1111/j.1524‐4733.2007.00168.x] - PubMed
Marsh 2014
Matteson 2013
Matteson 2015
Miller 2015
-
- Miller J, Lenhart G, Bonafede M, Basinski C, Lukes A, Troeger K. Cost effectiveness of endometrial ablation with the NovaSure((R)) system versus other global ablation modalities and hysterectomy for treatment of abnormal uterine bleeding: US commercial and Medicaid payer perspectives. International Journal of Womaens Health 2015;7:59‐73. [10.2147/ijwh.s75030] - PMC - PubMed
Munro 2011
-
- Munro MG, Critchley HO, Broder MS, Fraser IS, FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM‐COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. International Journal of Gynecology and Obstetrics 2011;113(1):3‐13. - PubMed
NICE 2018
-
- National Institute of Clinical Excellence. Heavy Menstrual Bleeding: Assessment and Management (NG88). London (UK): National Collaborating Centre for Women's and Children's Health, 2007 January (updated March 2018).
RCOG 2014
-
- Royal College of Obstetrics and Gynaecology. National heavy Menstrual Bleeding Audit/ Final Report. Royal College of Obstetrics and Gynaecology, London UK, 2014. [https://www.rcog.org.uk/globalassets/documents/guidelines/research‐‐audi...
Revman 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roumen 2007
-
- Roumen FJ. The contraceptive vaginal ring compared with the combined oral contraceptive pill: a comprehensive review of randomized controlled trials. Contraception 2007;75:420‐9. - PubMed
SIGN
-
- Scottish Intercollegiate Guidelines Network. Filters. www.sign.ac.uk/methodology/filters.html#random (accessed 26 July 2017).
SOGC 2013
-
- Society of Obstetricians and Gynaecologists of Canada. Abnormal uterine bleeding in pre‐menopausal women (Clinical practice guideline No 292). Journal of Obstetrics and Gynaecology Canada 2013;35(5 Suppl 1):S1‐S29. - PubMed
Stewart 2005
-
- Stewart FH, Kaunitz AM, Laguardia KD, Kervois DL, Fisher AC, Friedman AJ. Extended use of transdermal norelgestromin/ethinyl estradiol: a randomized trial. Obstetrics & Gynecology 2005;105:1389‐96. - PubMed
Uhm 2014
-
- Uhm S, Perriera L. Hormonal contraception as treatment for heavy menstrual bleeding: a systematic review. Clinical Obstetrics and Gynecology 2014;57(4):694‐717. - PubMed
References to other published versions of this review
Farquhar 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous