Prolonged Cold Ischemia Induces Necroptotic Cell Death in Ischemia-Reperfusion Injury and Contributes to Primary Graft Dysfunction after Lung Transplantation
- PMID: 30742487
- PMCID: PMC6670033
- DOI: 10.1165/rcmb.2018-0207OC
Prolonged Cold Ischemia Induces Necroptotic Cell Death in Ischemia-Reperfusion Injury and Contributes to Primary Graft Dysfunction after Lung Transplantation
Abstract
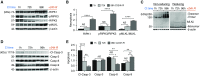
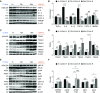
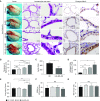
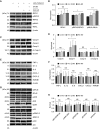
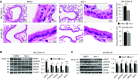
Primary graft dysfunction (PGD) is a major cause of morbidity and mortality after lung transplantation. Ischemia-reperfusion injury (IRI) is a key event that contributes to PGD, though complex interactions affect donor lungs status, such as preceding brain death (BD), hemorrhagic shock (HS), and pre-engraftment lung management, the latter recognized as important risk factors for PGD. We hypothesized that a multi-hit isogenic mouse model of lung transplantation is more closely linked to PGD than IRI alone. Left lung transplants were performed between inbred C57BL/6 mice. A one-hit model of IRI was established by inducing cold ischemia (CI) of the donor lungs at 0°C for 1, 72, or 96 hours before engraftment. Multi-hit models were established by inducing 24 hours of HS and/or 3 hours of BD before 24 hours of CI. The recipients were killed at 24 hours after transplant and lung graft samples were analyzed. In the one-hit model of IRI, up to 72-hour CI time resulted in minimal cellular infiltration near small arteries after 24-hour reperfusion. Extension of CI time to 96 hours led to increased cellular infiltration and necroptotic pathway activation, without evidence of apoptosis, after 24-hour reperfusion. In a multi-hit model of PGD, "HS + BD + IRI" demonstrated increased lung injury, cellular infiltration, and activation of necroptotic and apoptotic pathways compared with IRI alone. Treatment with an inhibitor of receptor-interacting protein kinase 1 kinase, necrostatin-1, resulted in a significant decrease of downstream necroptotic pathway activation in both single- and multi-hit models of IRI. Thus, activation of necroptosis is a central event in IRI after prolonged CI, though it may not be sufficient to cause PGD alone. Pathological evaluation of donor lungs after CI-induced IRI, in conjunction with pre-engraftment donor lung factors in our multi-hit model, demonstrated early evidence of lung injury consistent with PGD. Our findings support the premise that pre-existing donor lung status is more important than CI time alone for inflammatory pathway activation in PGD, which may have important clinical implications for donor lung retrieval.
Keywords: lung transplantation; necroptosis; primary graft dysfunction; reperfusion injury.
Figures


Comment in
-
Three Is Better than One: An Improved Multiple-Hit Model of Primary Graft Dysfunction.Am J Respir Cell Mol Biol. 2019 Aug;61(2):141-142. doi: 10.1165/rcmb.2019-0082ED. Am J Respir Cell Mol Biol. 2019. PMID: 30908931 Free PMC article. No abstract available.
Similar articles
-
Ischemia-reperfusion induces death receptor-independent necroptosis via calpain-STAT3 activation in a lung transplant setting.Am J Physiol Lung Cell Mol Physiol. 2018 Oct 1;315(4):L595-L608. doi: 10.1152/ajplung.00069.2018. Epub 2018 Jul 19. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 30024306
-
IRF1 and IL1A associated with PANoptosis serve as potential immune signatures for lung ischemia reperfusion injury following lung transplantation.Int Immunopharmacol. 2024 Sep 30;139:112739. doi: 10.1016/j.intimp.2024.112739. Epub 2024 Jul 28. Int Immunopharmacol. 2024. PMID: 39074415
-
Infusion of mesenchymal stem cells protects lung transplants from cold ischemia-reperfusion injury in mice.Lung. 2015 Feb;193(1):85-95. doi: 10.1007/s00408-014-9654-x. Epub 2014 Oct 26. Lung. 2015. PMID: 25344633
-
Role of the purinergic signaling network in lung ischemia-reperfusion injury.Curr Opin Organ Transplant. 2021 Apr 1;26(2):250-257. doi: 10.1097/MOT.0000000000000854. Curr Opin Organ Transplant. 2021. PMID: 33651003 Free PMC article. Review.
-
Primary Graft Dysfunction After Lung Transplantation.Clin Chest Med. 2017 Dec;38(4):641-654. doi: 10.1016/j.ccm.2017.07.005. Epub 2017 Sep 20. Clin Chest Med. 2017. PMID: 29128015 Review.
Cited by
-
Differentially expressed microRNAs in pre-transplant lung biopsies target immune checkpoint proteins and can predict primary graft dysfunction in lung transplantation.Heliyon. 2025 Feb 8;11(4):e42515. doi: 10.1016/j.heliyon.2025.e42515. eCollection 2025 Feb 28. Heliyon. 2025. PMID: 40028527 Free PMC article.
-
Primary graft dysfunction following lung transplantation: From pathogenesis to future frontiers.World J Transplant. 2023 Mar 18;13(3):58-85. doi: 10.5500/wjt.v13.i3.58. World J Transplant. 2023. PMID: 36968136 Free PMC article. Review.
-
Cross-Regulation of F-Box Protein FBXL2 with T-bet and TNF-α during Acute and Chronic Lung Allograft Rejection.J Immunol. 2022 Nov 1;209(9):1788-1795. doi: 10.4049/jimmunol.2200245. Epub 2022 Sep 16. J Immunol. 2022. PMID: 36113884 Free PMC article.
-
The novel compound PICCS improves cell viability during cold storage and reduces pulmonary ischemia-reperfusion injury in a rat model.Sci Rep. 2025 Apr 30;15(1):15217. doi: 10.1038/s41598-025-99851-7. Sci Rep. 2025. PMID: 40307436 Free PMC article.
-
Cold ischemia time alters cell-type specific senescence leading to loss of cellular integrity in mouse lungs.Exp Lung Res. 2024;50(1):184-198. doi: 10.1080/01902148.2024.2414974. Epub 2024 Oct 20. Exp Lung Res. 2024. PMID: 39427288
References
-
- Diamond JM, Arcasoy S, Kennedy CC, Eberlein M, Singer JP, Patterson GM, et al. Report of the International Society for Heart and Lung Transplantation Working Group on Primary Lung Graft Dysfunction, part II: epidemiology, risk factors, and outcomes—a 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36:1104–1113. - PubMed
-
- Gelman AE, Fisher AJ, Huang HJ, Baz MA, Shaver CM, Egan TM, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part III: mechanisms. A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36:1114–1120. - PMC - PubMed
-
- Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, et al. Ischemia–reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–1047. [Discussion, pp. 1047–1048.] - PubMed
-
- Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26:1004–1011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

